靶向TBXAS1:大豆苷元减轻apap诱导的肝损伤的新靶点
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
背景与目的:对乙酰氨基酚(APAP)过量是急性肝衰竭的主要原因。TBXAS1通过炎症细胞聚集、血管收缩和血栓形成等过程驱动炎症,被认为是催化血栓素A2生成的关键炎症调节剂。本研究旨在探讨生物活性化合物Daidzein (DAI)是否通过作用于TBXAS1对apap诱导的肝损伤具有肝保护作用。方法和结果:体外和体内实验显示,APAP诱导后肝脏TBXAS1水平升高。sirna介导的TBXAS1敲低可降低apap诱导的炎症和细胞毒性。网络药理学和转录组学分析发现TBXAS1是潜在的DAI靶点。在体内,DAI预处理可减轻apap诱导的小鼠肝损伤,降低TBXAS1水平。在体外,DAI预处理后再暴露APAP, AML-12细胞TBXAS1表达降低,炎症、氧化应激和细胞凋亡减少。机制分析表明,DAI对TBXAS1在体外和体内的表达均有调控作用。具体来说,DAI通过改变TBXAS1介导的TXA2水平来调节TLR4/NF-κB通路,从而保护apap诱导的损伤。结论:TBXAS1是DAI的新靶点。通过调节TBXAS1, DAI可以减轻apap诱导的肝损伤,包括肝细胞损伤、氧化应激、细胞凋亡和炎症。本研究为治疗apap引起的肝损伤提供了新的治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
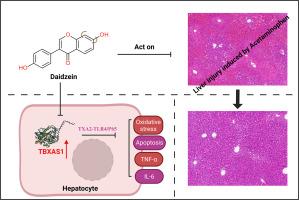
Targeting TBXAS1: a novel target of daidzein in alleviating APAP-induced hepatic injury
Background and Purpose
Acetaminophen (APAP) overdose is a major cause of acute liver failure. TBXAS1, driving inflammation via processes like inflammatory cell aggregation, vasoconstriction, and thrombosis, was picked out as a crucial inflammatory regulator that catalyzes thromboxane A2 generation. This study was conducted to explore whether Daidzein (DAI), a bioactive compound, has hepatoprotective effects against APAP-induced liver injury by acting on TBXAS1.
Methods and Results
In vitro and in vivo experiments revealed that hepatic TBXAS1 levels increased following APAP induction. SiRNA-mediated TBXAS1 knockdown reduced APAP-induced inflammation and cytotoxicity. Network pharmacology and transcriptomic analysis identified TBXAS1 as a potential DAI target. In vivo, DAI pretreatment mitigated APAP-induced liver injury in mice and lowered TBXAS1 levels. In vitro, DAI pretreatment followed by APAP exposure in AML-12 cells resulted in reduced TBXAS1 expression and decreased inflammation, oxidative stress, and apoptosis. Mechanistic analysis showed that DAI regulates TBXAS1 expression both in vitro and in vivo. Specifically, DAI modulates the TLR4/NF-κB pathway by altering TXA2 levels mediated by TBXAS1, thereby protecting against APAP-induced injury.
Conclusion
TBXAS1 is identified as a novel target of DAI. By modulating TBXAS1, DAI can reduce APAP-induced liver damage, including hepatocyte injury, oxidative stress, apoptosis, and inflammation. This study provides a new therapeutic approach for managing APAP-induced liver damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


