海绵/羟基磷灰石生物杂化骨再生支架的体内外综合评价
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-10-01
DOI:10.1016/j.bioadv.2025.214532
引用次数: 0
摘要
选择合适的支架是骨组织工程成功的关键。生物杂化支架,结合了合成聚合物的机械可调节性和天然材料的生物相容性,已引起广泛关注。在本研究中,利用从海绵胎儿肌(Sarcotragus foetidus, Sf)中分离出来的海绵制备生物杂交支架,模拟天然骨结构。羟基磷灰石(HAp)和掺硼羟基磷灰石(B-HAp)功能化了这些支架,以增强机械和骨传导性能。微计算机断层扫描(μCT)显示,所有组的互连孔隙率均在70%以上,有利于营养交换和细胞迁移。元素分析证实了碳、氧、钙和磷的存在,这些都是骨骼再生所必需的。力学测试表明,由于增强涂层的存在,Sf/HAp和Sf/B-HAp的杨氏模量分别达到了~ 20 kPa和~ 22 kPa。体外21天的实验表明,Sf/B-HAp支架具有最高的矿化、碱性磷酸酶(ALP)活性、胶原合成和细胞外基质(ECM)形成,表明其成骨能力增强。此外,抗菌试验显示Sf/B-HAp组具有较好的活性。使用大鼠颅骨骨缺损模型进行的体内研究显示,在所有组中,Sf/B-HAp支架的骨再生效果最为显著。总体而言,Sf基支架支持细胞粘附、增殖和分化,Sf/B-HAp变体具有优越的机械、骨导电性、骨诱导性和抗菌性能,使其成为高级骨组织工程的有希望的候选材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
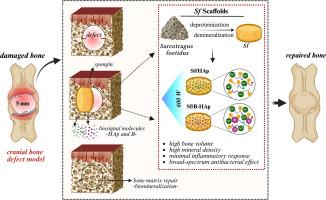
Comprehensive in vitro and in vivo assessment of spongin/hydroxyapatite biohybrid scaffolds for bone regeneration
The selection of an appropriate scaffold is vital for successful bone tissue engineering. Biohybrid scaffolds, combining the mechanical tunability of synthetic polymers with the biocompatibility of natural materials, have gained significant attention. In this study, biohybrid scaffolds were developed using spongin isolated from the marine sponge Sarcotragus foetidus (Sf), mimicking natural bone architecture. These scaffolds were functionalized with hydroxyapatite (HAp) and boron-doped HAp (B-HAp) to enhance mechanical and osteoconductive properties. Micro-computed tomography (μCT) revealed interconnected porosity above 70 % in all groups, facilitating nutrient exchange and cell migration. Elemental analysis confirmed the presence of carbon, oxygen, calcium, and phosphorus, essential for bone regeneration. Mechanical testing showed increased Young's modulus values, reaching ∼20 kPa for Sf/HAp and ∼ 22 kPa for Sf/B-HAp, due to the reinforcing coatings. In vitro assays over 21 days showed that the Sf/B-HAp scaffold exhibited the highest mineralization, alkaline phosphatase (ALP) activity, collagen synthesis, and extracellular matrix (ECM) formation, indicating enhanced osteogenic capacity. Additionally, antibacterial tests showed superior activity for the Sf/B-HAp group. In vivo studies conducted using a rat calvarial bone defect model over a 20-week period demonstrated significant bone regeneration across all groups, with the Sf/B-HAp scaffold exhibiting the most pronounced effect. Overall, Sf-based scaffolds supported cell adhesion, proliferation, and differentiation, with the Sf/B-HAp variant offering superior mechanical, osteoconductive, osteoinductive, and antibacterial properties—making it a promising candidate for advanced bone tissue engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


