交联密度对十二烷基改性阿拉斯加鳕鱼明胶微颗粒作为组织粘连止血粉的影响
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-10-03
DOI:10.1016/j.bioadv.2025.214534
引用次数: 0
摘要
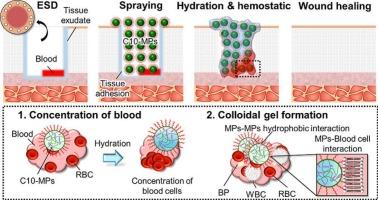
内镜下粘膜剥离术(ESD)是一种广泛应用于早期胃肠道肿瘤的微创治疗方法。然而,ESD术后出血仍然是主要的挑战。这种临床问题需要具有组织粘连和止血特性的生物材料。最近,我们开发了十二烷基修饰的阿拉斯加鳕鱼明胶微颗粒(C10-MPs)作为组织粘附止血粉末。干燥后的C10-MPs粘附于胃肠道组织表面并形成胶状凝胶,表现出优异的止血性能。然而,交联密度对组织粘连和止血性能的影响尚未明确。本文研究了交联密度对C10-MPs作为组织粘连止血粉性能的影响。通过改变热交联时间,制备了不同交联密度的C10-MPs。不同交联密度的C10-MPs均表现出快速水化。此外,具有较高交联密度的C10-MPs即使在潮湿环境中也表现出更高的粘附强度和水下粘附稳定性。相比之下,体外和体内止血评估模型显示,C10- mps热交联5 h (C10 5 h)是最合适的凝血能力粉。因此,C10 5 h在预防esd后出血方面具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of crosslinking densities on decanoyl-group modified Alaska pollock gelatin microparticles as tissue-adhesive hemostatic powders
Endoscopic submucosal dissection (ESD) is widely utilized as a minimally invasive treatment for early-stage gastrointestinal cancers. However, bleeding after ESD remains a major challenge. This clinical problem necessitates biomaterials with tissue-adhesive and hemostatic properties. Recently, we developed decanoyl group-modified Alaska pollock gelatin microparticles (C10-MPs) as tissue-adhesive hemostatic powders. Dried C10-MPs adhered to and formed colloidal gels on the gastrointestinal tissue surface and demonstrated excellent hemostatic properties. However, the effect of crosslinking density on tissue adhesion and hemostatic properties has not yet been clarified. Herein, we investigated the effect of the crosslinking density on C10-MPs' performance as tissue-adhesive hemostatic powders. C10-MPs with different crosslinking densities were prepared by varying the thermal crosslinking time. All C10-MPs with different crosslinking densities exhibited rapid hydration. Additionally, C10-MPs with higher crosslinking densities exhibited increased adhesion strength and underwater adhesion stability, even in moist environments. Contrastingly, in vitro and in vivo hemostatic evaluation models showed that C10-MPs thermally crosslinked for 5 h (C10 5 h) were the most suitable powder in terms of blood coagulation ability. Thus, C10 5 h has great potential for use in preventing post-ESD bleeding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


