煅烧CuMgAl-LDH高效脱除AB113染料:记忆效应和CCD-RSM优化
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本研究采用共沉淀法在pH为10的条件下合成了cumgal -层状双氢氧化物(LDH),制备出一定量的材料在823 K下煅烧5 h。结构和形态分析证实了成功合成的CuMgAl-LDH具有良好的结晶度,并且煅烧后形成了随机分布的开孔。合成的材料对偶氮染料酸性蓝113 (AB113)具有良好的去除效果,在10 mg、50 mg L−1、20 min、296±2 K条件下,吸附效率为98.1%。AB113对初始溶液pH、温度和共存离子的存在不敏感。另一方面,煅烧导致染料去除效率的显著提高,特别是在高浓度下。动力学和等温线模拟表明,实验数据与拟二阶模型和Langmuir模型拟合良好。热力学研究表明,该过程是自发的吸热过程。AB113染料在CuMgAl-LDH上的吸附机理涉及静电吸引和氢键相互作用。采用响应面法(RSM)中的中心复合设计(CCD)优化吸附过程的实验条件。影响影响最大的因素是CuMgAl-LDH质量(A)、AB113染料初始浓度(B)、二次项(A2, B2)和相互作用项(AB)。AB113染料在最佳条件(10 mg, 50 mg L−1,pH 8, 20 min, 298 K)下的吸附效率为98.67%,与批量实验结果吻合较好。由于其记忆效应,CuMgAl-LDH在连续6次重复使用循环中保持了较高的AB113染料去除效率。结果表明,CuMgAl-LDH是一种很有前途的脱除染料废水中污染物的吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
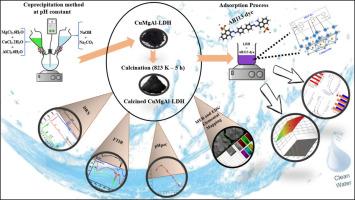
High-efficiency AB113 dye removal using calcined CuMgAl-LDH: memory effect and CCD-RSM optimization
In this study, CuMgAl-layered double hydroxide (LDH) was synthesized by co-precipitation at pH 10, an amount of the prepared material was calcined at 823 K for 5 h. Structural and morphological analyses confirmed the successful synthesis of CuMgAl-LDH with good crystallinity, and showed that calcination resulted in the formation of randomly distributed open pores. The synthesized material exhibited excellent performance in removing the azo dye Acid Blue 113 (AB113), achieving an adsorption efficiency of 98.1 % under batch conditions (10 mg, 50 mg L−1, 20 min, and 296 ± 2 K). The AB113 dye removal was not sensitive to the initial solution pH, temperature, and the presence of co-existing ions. On the other hand, Calcination led to a marked improvement in the dye removal efficiency, especially at high concentrations. Kinetic and isotherm modeling revealed that the experimental data fit well with pseudo-second-order and Langmuir model. The thermodynamic study indicates that the process was spontaneous and endothermic. Adsorption mechanism of AB113 dye onto CuMgAl-LDH involved electrostatic attraction and hydrogen bonding interactions. The central composite design (CCD) within response surface methodology (RSM) was used to optimize the experimental conditions for the adsorption process. The most influential factors were the CuMgAl-LDH mass (A), the initial concentration of AB113 dye (B), quadratic terms (A2, B2), and interaction term (AB). An adsorption efficiency of AB113 dye under optimal conditions (10 mg, 50 mg L−1, pH 8, 20 min, and 298 K) was 98.67 %, which is in good agreement with the batch experiments results. CuMgAl-LDH retained a high AB113 dye removal efficiency over six successive reuse cycles, owing to its memory effect. The results show that CuMgAl-LDH is a promising adsorbent for removing pollutants from dye wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


