通过界面疏水效应在水蒸气存在下促进微量甲烷光氧化
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
甲烷光氧化催化剂的耐水性研究对甲烷脱除具有重要意义和挑战性。在此,我们研究了水在不同金属(Cu, Pt)修饰的ZnO上光催化氧化微量甲烷的机理。我们发现抑制活性位点的水解离可以减轻羟基诱导的催化剂中毒。关键的见解表明,光产生的空穴(h+)介导钝化羟基(OH*)转化为活性羟基自由基(•OH),同时释放活性位点并促进反应。此外,温度相关实验表明,将温度从20℃提高到80 ℃,甲烷转化率提高了约4倍,证明了水分子从催化剂表面部分解吸释放出活性位点。实验结果表明,0.5 % Pt/ZnO的耐水性是0.5 % Cu/ZnO的2倍左右。这些发现为理解水介导的甲烷光氧化机理和合理设计高性能催化剂提供了宝贵的经验和指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
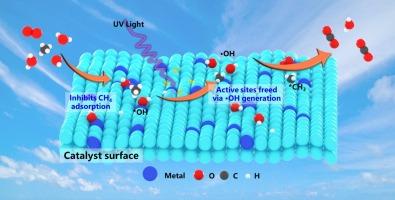
Boost of trace methane photo-oxidation in the presence of water vapor via the interfacial hydrophobic effect
The study of water resistance of methane photo-oxidation catalysts is important and challenging for the removal of methane from the atmosphere. Herein, we investigate the mechanistic role of water in the photocatalytic oxidation of trace methane on ZnO decorated with various metal (Cu, Pt). We found that suppressing water dissociation at active sites mitigates hydroxyl-induced catalyst poisoning. Critical insight reveals that photo-generated holes (h+) mediate the conversion of passivating hydroxyl groups (OH*) into reactive hydroxyl radicals (•OH), simultaneously liberating active sites and promoting the reaction. In addition, temperature-dependent experiments revealed that increasing the temperature from 20 to 80 °C enhanced the methane conversion by approximately 4-fold, proving that partial desorption of water molecules from the catalyst surface releases active sites. The experimental results show that 0.5 % Pt/ZnO is about 2-fold more water resistant than 0.5 % Cu/ZnO. These findings provide valuable experience and guidance for both mechanistic understanding of water-mediated methane photooxidation and rational design of high-performance catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


