双功能生物炭阴极对氯化乙烯无金属电催化脱氯:吸附耦合电催化还原机理
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
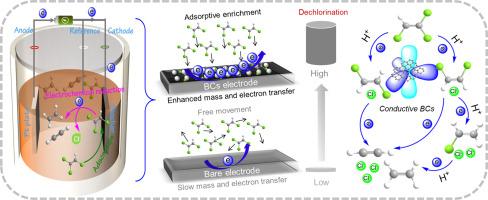
电化学还原脱氯为不需要化学添加剂的氯化乙烯(CEs)修复提供了一种环保、可持续的方法。然而,通常商用电极有限的质量和电子转移限制了与CEs的表面反应。本文利用具有高吸附容量(22.68 ~ 51.32 mg/g)和电导率(84 ~ 303 Ω)的无金属生物炭(BCs)作为阴极材料,对ce进行有效脱氯。BC900阴极对10 mg/L三氯乙烯(TCE)在24小时内的脱氯效率为98.14%,在-1.0 V的电位下,潜能值为0.161 h⁻¹。碳平衡和氯形态分析表明,几乎所有的C-Cl键都被劈裂,最终产物为乙烯和乙炔。淬火、动力学和电化学实验表明,脱氯过程是通过bc的导电结构进行的直接电子转移还原途径。此外,该催化剂对TCE脱氯的协同作用优于商用电催化剂,在环境水中具有较高的可重复使用性和广泛的适应性。这种方法也可以外推到其他ce,包括四氯乙烯、顺式-1,2-二氯乙烯和氯乙烯。这些发现突出了吸附耦合电化学还原脱氯机理,为开发高性能、生物质基环境电催化剂提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Metal-free electrocatalytic dechlorination of chlorinated ethenes using bifunctional biochar cathode: adsorption-coupled electrocatalytic reduction mechanism
Electrochemical reductive dechlorination provides an environmentally friendly and sustainable method for the remediation of chlorinated ethenes (CEs) without chemical additives. However, the limited mass and electron transfer of commonly commercial electrodes restricts surface reactions with CEs. Herein, metal-free biochars (BCs) with high adsorption capacity (22.68∼51.32 mg/g) and conductivity (84∼303 Ω) were utilized as cathode materials for the effective dechlorination of CEs. The BC900 cathode exhibited a dechlorination efficiency of 98.14% for 10 mg/L trichloroethylene (TCE) within 24 hours, with a kobs of 0.161 h⁻¹ under an applied potential of -1.0 V. Carbon balance and chloride form analyses indicated that nearly all C-Cl bonds were cleaved, with ethylene and acetylene identified as the primary end products. Quenching, kinetic, and electrochemical experiments demonstrated that the dechlorination process involves a direct electron transfer reduction pathway via conductive structures of BCs. Moreover, the synergistic effect on TCE dechlorination was superior to that of commercial electrocatalysts, achieving high reusability and wide adaptability in environmental water. This methodology can also be extrapolated to other CEs, including tetrachloroethylene, cis-1,2-Dichloroethylene, and vinyl chloride. These findings highlight an adsorption-coupled electrochemical reductive dechlorination mechanism for CEs, providing a theoretical foundation for the development of high-performance, biomass-based electrocatalysts in environmental applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


