用聚乙二醇螯合剂控制放射免疫偶联物的药代动力学
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
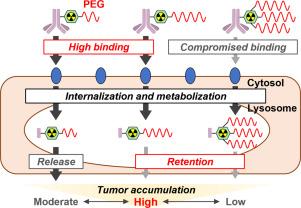
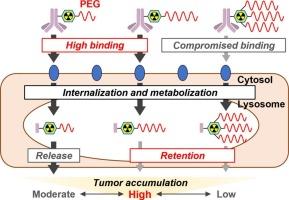
由单克隆抗体(mAb)和放射性同位素(RI)组成的放射免疫偶联物(RICs)在肿瘤学中提供了一种有吸引力的模式;然而,它们的低肿瘤/血液比率限制了它们的应用。为了在不影响肿瘤积累的情况下提高肿瘤/血液比例,我们开发了一种新的基于dota的螯合剂,该螯合剂含有马来酰亚胺和离散聚乙二醇(dPEG)支架,以合成可降解为肿瘤细胞中残留的高分子量放射代谢产物的RICs:由一个dPEG支架修饰的Mal-DOTA- peg24和Mal-DOTA-PEG48,以及由三个dPEG支架修饰的Mal-DOTA-(PEG48)3。基于曲妥珠单抗的含有Mal-DOTA- peg24、Mal-DOTA-PEG48或Mal-DOTA-(PEG48)3的RICs在人表皮生长因子受体2 (HER2)/表达new的SK-OV-3细胞上的放射性分布不同;含有高分子量聚乙二醇螯合剂的辐射代谢物在内化后在细胞中表现出更高水平的保留,但增加了与细胞表面的解离。含有一个dPEG48支架的RIC在体内的肿瘤放射性积累最高,并且在聚乙二醇化的RIC中具有良好的肿瘤/血液比,这表明优化的螯合剂聚乙二醇化显著有助于控制RICs的药代动力学。这些发现表明Mal-DOTA-PEG48作为放射合成平台具有良好的药代动力学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Control of pharmacokinetics of radioimmunoconjugates using chelating agents containing poly(ethylene glycol)
Radioimmunoconjugates (RICs) composed of a monoclonal antibody (mAb) and radioisotope (RI) offer an attractive modality in oncology; however, their low tumor/blood ratio has limited their utility. To establish a novel strategy to improve the tumor/blood ratio without compromising the tumor accumulation of RICs, we developed novel DOTA-based chelating agents containing maleimide and discrete poly(ethylene glycol) (dPEG) scaffolds to synthesize RICs degrading into residualizing radiometabolites with high molecular weight in tumor cells: Mal-DOTA-PEG24 and Mal-DOTA-PEG48 modified by one dPEG scaffold, and Mal-DOTA-(PEG48)3 modified by three dPEG scaffolds. Trastuzumab-based RICs with Mal-DOTA-PEG24, Mal-DOTA-PEG48, or Mal-DOTA-(PEG48)3 showed different radioactivity distribution on human epidermal growth factor receptor 2 (HER2)/neu-expressing SK-OV-3 cells; RICs containing a PEGylated chelating agent of higher molecular weight exhibited higher-level retention of radiometabolites in cells after their internalization but increased dissociation from cell surfaces. An RIC containing one dPEG48 scaffold showed the highest tumor accumulation of radioactivity in vivo with a favorable tumor/blood ratio among PEGylated RICs, indicating that the optimized PEGylation of chelating agents markedly contributes to controlling the pharmacokinetics of RICs. These findings suggest the promising property of Mal-DOTA-PEG48 as a radiosynthetic platform to develop RICs with favorable pharmacokinetics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


