用于连续间质液葡萄糖监测的热电驱动自供电微针传感器
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
个性化医疗中对持续、非侵入性生物标志物监测的需求加速了能源自主生物传感系统的发展。在此,我们提出了一种完全自供电、可穿戴的葡萄糖生物传感器,它集成了基于微针(MN)的电化学传感和柔性热电能量收集。该平台采用了一个贴合皮肤的热电发生器(TEG),该热电发生器由嵌入可拉伸的Ecoflex矩阵中的p-n碲化铋(Bi2Te3)热元件组成,可有效地将皮肤环境热梯度转化为电能。定制设计的小型化电压调节电路调节采集电压,提供稳定的0.4-0.8 V输出,足以在没有外部电源的情况下运行计时安培传感模块。MN工作电极采用分层石墨烯界面和保形双金属Au/Pt薄膜设计,以提高电活性表面积,电子转移动力学和酶固定化能力。葡萄糖氧化酶(GOx)随后固定在电极表面,使葡萄糖选择性酶氧化,产生可量化的电化学信号。在三种被评估的MN几何形状中,1毫米尖端高度的配置表现出最佳的皮肤界面和电化学性能,产生最高的电流响应。在人工间质液中进行的体外评估表明,葡萄糖的线性检测范围为4-24 mM,灵敏度高,对干扰物的交叉反应性最小。在大鼠模型中的体内验证证实了与血糖水平的强相关性,证明了有效的糖尿病监测,出色的生物相容性和强大的组织整合。这项工作为自我维持、强大的无创血糖监测提供了一个潜在的平台,并为个性化医疗中的下一代可穿戴生物传感器奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
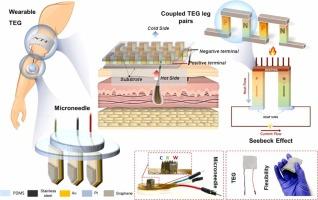
Thermoelectric-driven self-powered microneedle sensor for continuous interstitial fluid glucose monitoring
The demand for continuous, non-invasive biomarker monitoring in personalized healthcare has accelerated the development of energy-autonomous biosensing systems. Herein, we present a fully self-powered, wearable glucose biosensor that integrates microneedle (MN)-based electrochemical sensing with flexible thermoelectric energy harvesting. The platform employs a skin-conformable thermoelectric generator (TEG) composed of p-n bismuth telluride (Bi2Te3) thermoelements embedded within a stretchable Ecoflex matrix, which effectively converts skin-ambient thermal gradients into electrical power. A custom-designed miniaturized voltage regulation circuit regulates the harvested voltage, delivering a stable 0.4-0.8 V output sufficient to operate a chronoamperometric sensing module without external power sources. The MN working electrode is engineered with a hierarchical graphene interface and a conformal bimetallic Au/Pt thin film to enhance electroactive surface area, electron transfer kinetics, and enzyme immobilization capacity. Glucose oxidase (GOx) is subsequently immobilized onto the electrode surface to enable selective enzymatic oxidation of glucose, generating a quantifiable electrochemical signal. Among three evaluated MN geometries, the 1 mm tip height configuration demonstrated optimal dermal interfacing and electrochemical performance, yielding the highest current response. In vitro assessments in artificial interstitial fluid demonstrated a linear detection range of 4-24 mM glucose with high sensitivity and minimal cross-reactivity to interfering analytes. In vivo validation in a rat model confirmed strong correlation with blood glucose levels, demonstrating effective diabetes monitoting, excellent biocompatibility, and robust tissue integration. This work presents a potential platform for self-sustained, robust non-invasive glucose monitoring and sets a foundation for next-generation wearable biosensors in personalized medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


