时空温度控制的全息加热显微镜揭示细胞热敏钙信号
IF 5.4
2区 工程技术
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
光学微加热技术揭示了生物系统如何在微观尺度上感知加热和冷却。传感是基于热敏生化反应,经常参与膜蛋白,Ca2+通道和泵转换传感信息作为细胞中的Ca2+信号。这些发现强调了热操纵细胞内Ca2+信号的可行性。然而,热敏Ca2+信号如何表现,特别是在多细胞系统中,仍然难以捉摸。在本研究中,为了扩展光学微加热技术的时空温度控制能力,我们提出了全息加热显微镜。利用硅空间光调制器(LCOS-SLM)上的反射液晶对可吸水的红外激光进行调制。显示在LCOS-SLM上的计算机生成全息图调制红外激光的空间相位模式,以在显微镜焦平面上产生预先设计的温度梯度。全息加热显微镜显示了热敏Ca2+信号是如何在MDCK细胞、大鼠海马神经元和大鼠新生心肌细胞中产生和传播的。此外,时间温度梯度的光学控制揭示了HeLa细胞中Ca2+信号的冷却速率依赖性。这些发现证明了全息加热显微镜在研究细胞热敏性和热操纵细胞功能方面的扩展能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
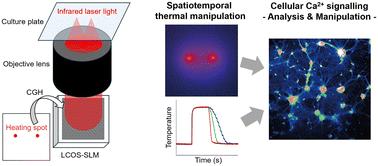
Spatiotemporal temperature control by holographic heating microscopy unveils cellular thermosensitive calcium signalling
Optical microheating technologies have revealed how biological systems sense heating and cooling at the microscopic scale. Sensing is based on thermosensitive biochemical reactions that frequently engage membrane proteins, Ca2+ channels, and pumps to convert sensing information as the Ca2+ signalling in cells. These findings highlight the feasibility of thermally manipulating intracellular Ca2+ signalling. However, how the thermosensitive Ca2+ signalling would behave, particularly in multicellular systems, remains elusive. In this study, to extend the ability of the spatiotemporal temperature control by optical microheating technologies, we propose holographic heating microscopy. Water-absorbable infrared (IR) laser light is modulated by a reflective liquid crystal on a silicon spatial light modulator (LCOS-SLM). A computer-generated hologram displayed on the LCOS-SLM modulates the spatial phase pattern of the IR laser light to generate predesigned temperature gradients at the microscope focal plane. The holographic heating microscopy visualises how thermosensitive Ca2+ signalling is generated and propagated in MDCK cells, rat hippocampal neurons, and rat neonatal cardiomyocytes. Moreover, the optical control of the temporal temperature gradient reveals the cooling-rate dependency of Ca2+ signalling in HeLa cells. These findings demonstrate the extended ability of holographic heating microscopy in investigating cellular thermosensitivities and thermally manipulating cellular functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Lab on a Chip
工程技术-化学综合
CiteScore
11.10
自引率
8.20%
发文量
434
审稿时长
2.6 months
期刊介绍:
Lab on a Chip is the premiere journal that publishes cutting-edge research in the field of miniaturization. By their very nature, microfluidic/nanofluidic/miniaturized systems are at the intersection of disciplines, spanning fundamental research to high-end application, which is reflected by the broad readership of the journal. Lab on a Chip publishes two types of papers on original research: full-length research papers and communications. Papers should demonstrate innovations, which can come from technical advancements or applications addressing pressing needs in globally important areas. The journal also publishes Comments, Reviews, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


