cucl2介导的C(sp3)- h和C(sp3)- o氧化/醚双吲哚酰化-双吲哚基甲烷衍生物的合成
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在廉价和容易获得的CuCl2的参与下,在没有配体和氧化剂的情况下,开发了可见光诱导的吲哚和脂肪醚的串联C(sp3)-H氧化,C(sp3)-O裂解和Friedel - Crafts烷基化反应,提供了合成有用的3,3 ' -双吲哚甲烷衍生物,收率中等至较高。这种转化具有广泛的底物范围和良好的官能团相容性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
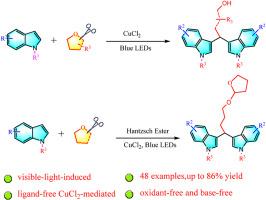
Visible-light-enabled CuCl2-mediated tandem C(sp3)-H and C(sp3)-O oxidant/bisindolylation of ethers – Synthesis of bisindolylmethane derivatives
Under the participation of cheap and readily available CuCl2, a visible light-induced tandem C(sp3)-H oxidation, C(sp3)-O cleavage and Friedel−Crafts alkylation of indoles and aliphatic ethers in the absence of ligand and oxidants is developed, affording synthetically useful 3,3′-bisindolylmethane derivatives in moderate to good yields. This transformation exhibits broad substrate scope and good functional group compatibility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


