丙二腈-异硫氰酸酯(或异硒氰酸酯)加合物开环法合成新的2-(噻唑-2-乙基)丙二腈和2-(1,3-硒唑-2-乙基)丙二腈衍生物
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
结合近年来对五元杂环化合物合成的研究,本研究以硝基环氧化物为高效起始原料,通过开环法制备了2-(1,3-硒化唑-2-酰基)丙二腈和2-(噻唑-2-酰基)丙二腈的新型衍生物。该反应是通过生成丙二腈-异硫氰酸酯(或异硒氰酸酯)加合物,然后加入硝基环氧化物合成产物,纯化容易。通过克级实验,在室温和丙酮溶剂中进行四组分反应,在不添加催化剂的情况下,证明了该过程的有效性。合成并鉴定了18个新的1,3-硒化唑和噻唑类杂环化合物,并通过IR、Mass和NMR分析对其进行了证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
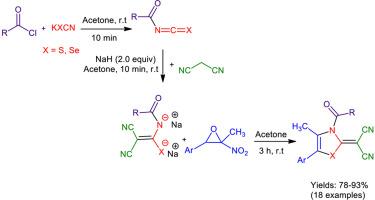
Synthesis of new 2-(thiazol-2-ylidene) malononitrile, and 2-(1,3-selenazol-2-ylidene) malononitrile derivatives via nitroepoxide ring opening with malononitrile-isothiocyanate (or isoselenocyanate) adducts
In line with recent research on the synthesis of five-membered heterocyclic compounds, the synthesis of novel derivatives of 2-(1,3-selenazol-2-ylidene) malononitrile and 2-(thiazol-2-ylidene) malononitrile was carried out via the ring opening of nitroepoxide as an efficient starting material in this study. This reaction was carried out via the formation of malononitrile-isothiocyanate (or isoselenocyanate) adducts, followed by the addition of nitroepoxide to synthesize the products with easy purification. The effectiveness of this process was demonstrated through a gram-scale experiment, performing the four-component reaction, without the addition of a catalyst, at room temperature and in acetone solvent. 18 new heterocyclic compounds from the new 1,3-selenazoles and thiazoles family were synthesized and identified, and confirmed by IR, Mass, and NMR analyses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


