受保护的环氧醇重排成四氢呋喃衍生物:保护基团很重要!
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们探索了一个有趣的将受保护的环氧醇重排成四氢呋喃衍生物的方法。我们的方案操作简单,涉及用催化量的三氟化硼二乙醚在二氯甲烷中处理底物,无需任何特殊预防措施以排除空气或环境水分。保护基的性质决定了环化的立体化学结果。例如,反式二取代环氧化物带有垂坠酯或氨基甲酸酯,重排得到具有连续立体中心的四氢呋喃。对于这些底物,我们假设在酯或氨基甲酸酯的羰基氧攻击环氧化物时开始转化。相反,对于含有游离醇或醚的反式二取代环氧化物,环化得到具有反构型的连续立体中心的四氢呋喃。这里,我们认为发生了简单的SN2对环氧化物的攻击。我们还研究了与氮啶醇及其衍生物和氧烷酯的环化反应,发现其中一些底物与反应条件是相容的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
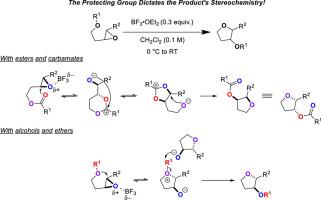
Rearrangement of protected epoxy-alcohols into tetrahydrofuran derivatives: The protecting group matters!
We have explored an interesting rearrangement of protected epoxy-alcohols into tetrahydrofuran derivatives. Our protocol is operationally simple and involves treatment of a substrate with catalytic quantities of boron trifluoride diethyl etherate in methylene chloride without any special precautions to exclude air or ambient moisture. The nature of the protecting group dictates the stereochemical outcome of the cyclization. For example, with trans-di-substituted epoxides bearing pendant esters or carbamates, the rearrangement gives tetrahydrofurans with contiguous stereocenters in a syn configuration. With these substrates, we hypothesize that the transformation initiates upon attack of the epoxide by the carbonyl oxygen of the ester or carbamate. Conversely, with trans-di-substituted epoxides bearing free alcohols or ethers, cyclization gives tetrahydrofurans with contiguous stereocenters in an anti configuration. Here, we believe that a simple SN2 attack on the epoxide is taking place. We also examined the cyclization with aziridine alcohols and their derivatives and with oxetane esters and found that some of these substrates were compatible with the reaction conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


