苯恶唑啉类:合成、结构、光谱、氧化还原和理论研究
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
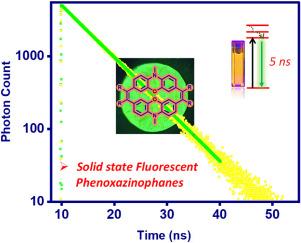
以市售的苯恶嗪为原料,经三步合成了三种荧光苯恶嗪,每一种都含有两个由两个乙烯连接剂桥接的苯恶嗪单元。在乙烯桥上首次引入甲基和苯基等取代基,使得苯恶唑啉类化合物的电子性质发生了显著的改变。x射线结构表明,苯恶嗪中苯恶嗪部分呈蝴蝶状非平面结构,两个苯环平面之间的二面角(36.7°)比游离苯恶嗪中的二面角(167°)明显减小。光谱和电化学研究表明,吩恶唑啉的性质与先前报道的吩噻吩啉明显不同,并取决于桥接乙烯碳上取代基的类型。值得注意的是,苯恶唑啉类化合物在450 ~ 650 nm范围内表现出固体绿色荧光,发射光谱较宽,量子产率在0.32 ~ 0.35之间。初步研究表明,苯恶唑啉类化合物表现为聚集诱导发射。电化学研究表明,苯恶唑啉类化合物具有高富电子性质,DFT/TD-DFT研究结果与实验结果一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phenoxazinophanes: Synthesis, structure, spectral, redox and theoretical studies
Three fluorescent phenoxazinophanes, each incorporating two phenoxazine units bridged by two ethene linkers, were synthesized in a three-step sequence starting from commercially available phenoxazine. Substituents such as methyl and phenyl groups were introduced at the ethene bridges for the first time, resulting in significant modulation of the electronic properties of phenoxazinophanes. The X-ray structure revealed that phenoxazine moiety in phenoxazinophane exhibits a butterfly-shaped, non-planar structure with a significant reduction in dihedral angle (36.7°) between the planes of the two benzene rings compared to the angle in free phenoxazine (167°). The spectroscopic, and electrochemical studies revealed that the properties of phenoxazinophanes markedly distinct from those of previously reported phenothiazinophanes and depends on the type of substituents present at the bridged ethene carbons. Remarkably, the phenoxazinophanes exhibit green fluorescence in the solid state with a broad emission in the region of 450–650 nm and quantum yields were in the range of 0.32–0.35. Preliminary studies indicated that phenoxazinophanes exhibit aggregation-induced emission. The electrochemical studies revealed that phenoxazinophanes are highly electron rich and DFT/TD-DFT studies were in agreement with the experimental observations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


