氮氧呋喃嘧啶的易氧化还原合成
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了一种有效的两步氧化还原方法,通过化学选择性还原现成的4-硝基呋喃嘧啶,然后通过naio4介导氧化偶联从而形成羟胺,制备偶氮氧呋喃嘧啶。所建立的方案操作简单,不需要详尽的色谱纯化,并且能够制备范围广泛的目标偶氮氧呋喃嘧啶。此外,我们的方法也适用于合成其他具有代表性的含呋喃氮、吡啶或苄基的偶氮基化合物,证实了它在有机化学中的合成潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
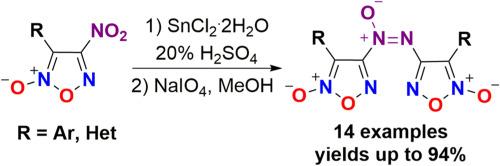
Facile redox synthesis of azoxyfuroxans
Herein, we report on an efficient two-step redox approach for the preparation of azoxyfuroxans via chemoselective reduction of the readily available 4-nitrofuroxans followed by NaIO4-mediated oxidative coupling of thus formed hydroxylamines. The established protocol is operationally simple, does not require exhaustive chromatography purification and enables a preparation of a wide range of target azoxyfuroxans. Additionally, our method is suitable for the synthesis of other representative azoxy compounds incorporating furazan, pyridine or benzyl moieties, confirming its synthetic potential in organic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


