nhc催化获得四环八元内酯的高阶(5 + 3)环化反应
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了在Sc(OTf)3的催化下,以2-吲哚酚和α,β-炔醇为起始原料,由n -杂环羰基(NHC)催化的高阶(5 + 3)环化反应,可直接合成含桥接芳基-吲哚单元的8元四环内酯,产率中高。该方案具有广泛的底物范围,与取代基的良好相容性和温和的条件,为创建中等大小的内酯提供了模块化的有机催化方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
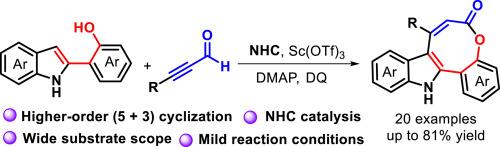
NHC-catalyzed higher-order (5 + 3) cyclization for accessing tetracyclic eight-membered lactones
An oxidative N-heterocyclic carbene (NHC)-catalyzed higher-order (5 + 3) cyclization reaction starting from 2-indolylphenols and α,β-alkynals with the aid of Sc(OTf)3 is reported, enabling the direct synthesis of tetracyclic 8-membered lactones bearing a bridged aryl-indole unit in moderate to good yields. This protocol exhibits a broad substrate scope, good compatibility with substituents and mild conditions, offering a modular organocatalytic approach for creating medium-sized lactones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


