以蒸氨废液为钙源,乙醇对文石形成和生长的影响
IF 4.6
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
文石是最独特的无水碳酸钙(CaCO3)晶体之一,它是通过多个步骤形成的。乙醇的存在会影响无水CaCO3结晶的析出途径和形貌。在这项研究中,我们研究了温度和搅拌对文石在乙醇基体系中的物理化学性质和形成过程的影响。用x射线衍射和扫描电镜对所得文石颗粒进行了表征。通过密度泛函理论和分子动力学模拟进一步研究了乙醇在文石生长中的作用。结果表明,乙醇的存在使文石含量显著增加,形成单相文石。温度和搅拌改变了乙醇在反应体系中的分布以及与钙离子碰撞的可能性,从而影响文石的成核和生长。机理分析表明,乙醇通过调节文石晶体与钙离子的络合以及与晶体表面的多种相互作用来调控文石晶体的成核和生长。这些影响最终决定了文石晶体的含量和性能。本研究介绍了一种利用工业废液生产高附加值文石型碳酸钙产品的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
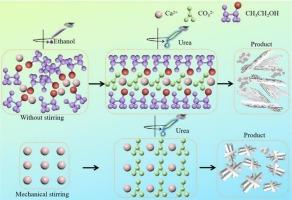
Effects of ethanol on aragonite formation and growth used steamed ammonia liquid waste as calcium sources
Aragonite, one of the most distinctive anhydrous calcium carbonate (CaCO3) crystals, forms via a multistep process. The presence of ethanol can influence the precipitation pathways and morphology of anhydrous crystalline CaCO3 polymorphs. In this study, we investigated the effects of temperature and agitation on the physicochemical properties and formation process of aragonite in an ethanol-based system. The resulting aragonite particles were characterized using X-ray diffraction and scanning electron microscopy. The role of ethanol in aragonite growth was further examined through density functional theory and molecular dynamics simulations. The results showed that the presence of ethanol considerably increased the aragonite content, leading to the formation of single-phase aragonite. Temperature and agitation altered the distribution of ethanol within the reaction system and the likelihood of collisions with calcium ions, thereby influencing the nucleation and growth of aragonite. Mechanistic analysis indicated that ethanol regulated the nucleation and growth of aragonite crystals by modulating their complexation with calcium ions and interacting with crystal surfaces in various ways. These effects ultimately determined the content and performance of the resulting aragonite crystals. This study introduced a novel approach for producing high-value-added aragonite-type CaCO3 products from industrial waste streams.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Powder Technology
工程技术-工程:化工
CiteScore
9.90
自引率
15.40%
发文量
1047
审稿时长
46 days
期刊介绍:
Powder Technology is an International Journal on the Science and Technology of Wet and Dry Particulate Systems. Powder Technology publishes papers on all aspects of the formation of particles and their characterisation and on the study of systems containing particulate solids. No limitation is imposed on the size of the particles, which may range from nanometre scale, as in pigments or aerosols, to that of mined or quarried materials. The following list of topics is not intended to be comprehensive, but rather to indicate typical subjects which fall within the scope of the journal's interests:
Formation and synthesis of particles by precipitation and other methods.
Modification of particles by agglomeration, coating, comminution and attrition.
Characterisation of the size, shape, surface area, pore structure and strength of particles and agglomerates (including the origins and effects of inter particle forces).
Packing, failure, flow and permeability of assemblies of particles.
Particle-particle interactions and suspension rheology.
Handling and processing operations such as slurry flow, fluidization, pneumatic conveying.
Interactions between particles and their environment, including delivery of particulate products to the body.
Applications of particle technology in production of pharmaceuticals, chemicals, foods, pigments, structural, and functional materials and in environmental and energy related matters.
For materials-oriented contributions we are looking for articles revealing the effect of particle/powder characteristics (size, morphology and composition, in that order) on material performance or functionality and, ideally, comparison to any industrial standard.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


