综合蛋白质组学和代谢组学分析显示,脂质代谢失调和铁下沉是妊娠肝内胆汁淤积中胎盘功能障碍的潜在驱动因素
IF 3.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular and cell biology of lipids
Pub Date : 2025-10-03
DOI:10.1016/j.bbalip.2025.159692
引用次数: 0
摘要
妊娠肝内胆汁淤积症(ICP)与不良胎儿结局相关,而目前的生物标志物,如总胆汁酸仍不理想。本研究旨在通过综合代谢组学和蛋白质组学分析,鉴定新的生物标志物,阐明ICP的代谢途径。从ICP模型大鼠和健康对照中获得胎盘剖面图,在人类胎盘和血清样本中验证了差异代谢物和蛋白质。多组学整合揭示了明显的脂质代谢失调,特别是脂肪酸降解和生物合成,突出了脂质在ICP中的核心作用。棕榈酸和酰基辅酶a合成酶长链家族成员1 (ACSL1)是这些途径的核心,在ICP中显著升高,具有较高的诊断价值(曲线下面积0.794和0.825),联合检测达到0.894。这两种标志物还根据疾病严重程度对患者进行分层,表明它们在疾病监测和风险分类方面的潜在用途。此外,在患者胎盘组织和牛磺胆酸(TCA)处理的滋养细胞中证实,铁下垂与ICP病理生理有关,显示谷胱甘肽过氧化物酶4 (GPX4)和溶质载体家族7成员11 (SLC7A11)减少,前列腺3 (STEAP3)、转铁蛋白受体蛋白1 (CD71)和酰基辅酶a合成酶长链家族4 (ACSL4)的六跨膜上皮抗原增加。总之,棕榈酸和ACSL1是ICP诊断和分类的有希望的生物标志物,而铁下垂有助于ICP相关的胎盘功能障碍。这些发现提供了将脂质代谢改变和铁下垂与ICP联系起来的全面证据,为临床诊断和潜在的治疗策略提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
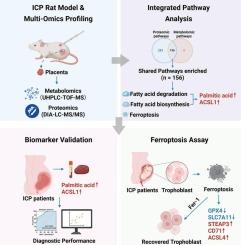
Integrated proteomic and metabolomic analysis reveals lipid metabolic dysregulation and ferroptosis as potential drivers of placental dysfunction in intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP) is associated with adverse fetal outcomes, while current biomarkers such as total bile acid remain suboptimal. This study aimed to identify novel biomarkers and clarify metabolic pathways underlying ICP through integrated metabolomic and proteomic analyses. Placental profiles were obtained from ICP model rats and healthy controls, with differential metabolites and proteins validated in human placental and serum samples. Multiomics integration revealed prominent dysregulation of lipid metabolism, particularly fatty acid degradation and biosynthesis, highlighting lipids as central players in ICP. Palmitic acid and acyl-CoA synthetase long chain family member 1 (ACSL1) were central to these pathways, markedly elevated in ICP, and showed high diagnostic value (area under the curve 0.794 and 0.825), with combined detection reaching 0.894. Both markers also stratified patients by disease severity, suggesting their potential use for disease monitoring and risk classification. Moreover, ferroptosis was implicated in ICP pathophysiology, supported by validations in both patient placental tissues and taurocholic acid (TCA)-treated trophoblast cells, showing reduced glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11) together with increased six-transmembrane epithelial antigen of prostate 3 (STEAP3), transferrin receptor protein 1 (CD71), and acyl-CoA synthetase long-chain family member 4 (ACSL4). In summary, palmitic acid and ACSL1 represent promising biomarkers for ICP diagnosis and classification, while ferroptosis contributes to ICP-related placental dysfunction. These findings provide comprehensive evidence linking altered lipid metabolism and ferroptosis to ICP, offering new insights for clinical diagnosis and potential therapeutic strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.00
自引率
2.10%
发文量
109
审稿时长
53 days
期刊介绍:
BBA Molecular and Cell Biology of Lipids publishes papers on original research dealing with novel aspects of molecular genetics related to the lipidome, the biosynthesis of lipids, the role of lipids in cells and whole organisms, the regulation of lipid metabolism and function, and lipidomics in all organisms. Manuscripts should significantly advance the understanding of the molecular mechanisms underlying biological processes in which lipids are involved. Papers detailing novel methodology must report significant biochemical, molecular, or functional insight in the area of lipids.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


