PELT共聚酯的高效单体循环利用:理论和实验方法的相互作用
IF 7.4
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
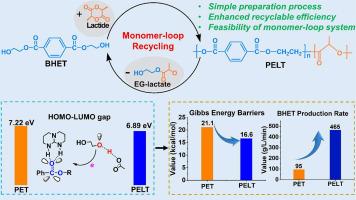
聚对苯二甲酸乙酯-共乳酸(PELT)共聚酯,含有可再生和可生物降解的成分,为提高PET的可回收性提供了一条很有前途的途径。然而,PELT的化学单体循环利用尚未得到研究。本文通过理论和实验相结合的方法,报道了一种高效的PELT单体循环回收方法。密度泛函理论计算揭示了这种循环过程的可行性,并确定HOCH2CH2O对PELT链的羰基碳的亲核攻击是决定速度的步骤。速率决定步骤的电荷转移分析表明,PELT的HOMO-LUMO间隙明显小于PET,导致PELT分解的吉布斯能垒较低(16.6 kcal/mol vs 21.1 kcal/mol)。以对苯二甲酸双(羟乙基)酯(BHET)为起始剂,通过开环聚合,缩聚合成了中等分子量的PELT。实验分析表明,丙交酯的掺入改变了晶体结构,降低了玻璃化转变温度、结晶温度和熔融温度。以PELT-30为样品,在HTBD-OAc催化下,190℃,60分钟后,化学回收实验获得了91.5%的BHET收率。降解产物被成功地重新聚合成PELT,其性能与原始材料相当,证实了单体环回收方法的可行性。生命周期评估表明,与PET相比,PELT在闭环回收过程中减少了温室气体(GHG)排放和一次能源需求(PED)。这项工作促进了聚酯的可持续回收,并有助于开发更环保的材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient monomer-loop recycling of PELT copolyesters: Interplay of theoretical and experimental approaches
Poly(ethylene terephthalate-co-lactic acid) (PELT) copolyesters, incorporating renewable and biodegradable components, offer a promising route to enhance the recyclability of PET. However, the chemical monomer-loop recycling of PELT has not been investigated. Here, an efficient monomer-loop recycling of PELT was reported through a combination of theoretical and experimental approach. Density functional theory calculations reveal the feasibility of this recycling process and identify the nucleophilic attack of HOCH2CH2O⁻ on the carbonyl carbon of the PELT chain as the rate-determining step. Charge transfer analysis on the rate-determining step shows that PELT has a significantly smaller HOMO-LUMO gap than PET, leading to a lower Gibbs energy barrier for PELT decomposition (16.6 kcal/mol versus 21.1 kcal/mol). Experimentally, moderate molecular weight PELT was synthesized via ring-opening polymerization of lactide initiated by bis(hydroxyethyl) terephthalate (BHET) followed by polycondensation. Experimental analyses demonstrated that lactide incorporation alters the crystalline structure, which reduces the glass transition temperature, crystallization temperature, and melting temperature. Using PELT-30 as a sample, chemical recycling experiments achieved a 91.5 % BHET yield after 60 minutes at 190°C under HTBD-OAc catalysis. The degradation products were successfully repolymerized into PELT with properties comparable to the original material, confirming the viability of the monomer-loop recycling approach. Life-cycle assessment demonstrated that PELT reduces greenhouse gas (GHG) emissions and primary energy demand (PED) during closed-loop recycling compared with PET. This work advances the sustainable recycling of polyesters and contributes to the development of more eco-friendly materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Degradation and Stability
化学-高分子科学
CiteScore
10.10
自引率
10.20%
发文量
325
审稿时长
23 days
期刊介绍:
Polymer Degradation and Stability deals with the degradation reactions and their control which are a major preoccupation of practitioners of the many and diverse aspects of modern polymer technology.
Deteriorative reactions occur during processing, when polymers are subjected to heat, oxygen and mechanical stress, and during the useful life of the materials when oxygen and sunlight are the most important degradative agencies. In more specialised applications, degradation may be induced by high energy radiation, ozone, atmospheric pollutants, mechanical stress, biological action, hydrolysis and many other influences. The mechanisms of these reactions and stabilisation processes must be understood if the technology and application of polymers are to continue to advance. The reporting of investigations of this kind is therefore a major function of this journal.
However there are also new developments in polymer technology in which degradation processes find positive applications. For example, photodegradable plastics are now available, the recycling of polymeric products will become increasingly important, degradation and combustion studies are involved in the definition of the fire hazards which are associated with polymeric materials and the microelectronics industry is vitally dependent upon polymer degradation in the manufacture of its circuitry. Polymer properties may also be improved by processes like curing and grafting, the chemistry of which can be closely related to that which causes physical deterioration in other circumstances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


