基于界面工程的一维自支撑NiFe2O4/NiMoO4异质结构双功能电催化剂高效分解海水
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
海水直接电解是一种很有前途的制氢方法。然而,由于盐的高浓度和氯的竞争反应,开发高效、经济的电催化剂仍然是工业级电流密度海水电解的一个重大挑战。本文通过简单的水热浸渍和煅烧的方法,将NiMoO4纳米线与NiFe2O4纳米颗粒结合在碳毡(CF)上,构建了NiFe2O4/NiMoO4一维异质结构作为海水整体分裂的双功能电催化剂。在碱性海水(1 mol/L KOH + 0.5 mol/L NaCl)中,在400 mA/cm2下析氧反应和析氢反应的过电位分别为237和292 mV,这是由于NiFe2O4/NiMoO4丰富的界面暴露了更多的活性位点,扩大了活性表面积,从而提高了电催化剂的固有活性,促进了反应动力学。值得注意的是,它在碱性海水中表现出1.95 V的低电压,可驱动400 mA/cm2的电流密度,在100 mA/cm2以上时具有200 h的优异稳定性,表现出优异的性能和良好的耐腐蚀性。这项工作为构建高效、经济的工业海水电解电催化剂提供了有效的策略,强调了其在可持续能源应用方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
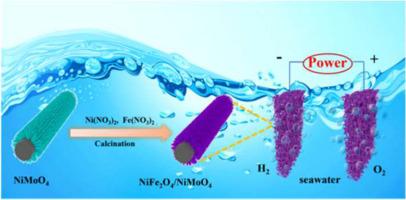
1D self-supporting NiFe2O4/NiMoO4 heterostructure as bifunctional electrocatalyst via interface engineering for highly efficient seawater splitting
Direct seawater electrolysis is a promising way for hydrogen energy production. However, developing efficient and cost-effective electrocatalysts remains a significant challenge for seawater electrolysis with industrial-level current density due to high concentration of salts and compete reaction of chlorine evolution. Herein, a 1D NiFe2O4/NiMoO4 heterostructure as a bifunctional electrocatalyst for overall seawater splitting is constructed by combining NiMoO4 nanowires with NiFe2O4 nanoparticles on carbon felt (CF) by a simple hydrothermal, impregnation and calcination method. The electrocatalyst exhibits low overpotential of 237 and 292 mV for oxygen evolution reaction and hydrogen evolution reaction at 400 mA/cm2 in the alkaline seawater (1 mol/L KOH + 0.5 mol/L NaCl) due to the plentiful interfaces of NiFe2O4/NiMoO4 which exposes more active sites and expands the active surface area, thereby enhancing its intrinsic activity and promoting the reaction kinetics. Notably, it displays low voltages of 1.95 V to drive current density of 400 mA/cm2 in alkaline seawater with its excellent stability of 200 h at above 100 mA/cm2, exhibiting outstanding performance and good corrosion resistance. This work provides an effective strategy for constructing efficient and cost-effective electrocatalysts for industrial seawater electrolysis, underscoring its potential for sustainable energy applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


