调节多孔碳中的石墨畴以增强苯在丙酮上的竞争性吸附:石墨化在分离选择性中的关键作用
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
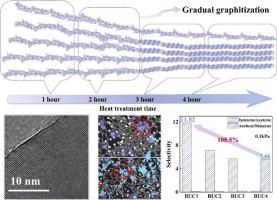
多孔活性炭具有可调节的表面化学性质、良好的孔隙度和环境相容性,其高表面积为挥发性有机化合物(VOC)的吸附提供了丰富的活性位点。本研究以苯并咪唑为碳前体,高铁酸钾为活化剂。我们创新性地利用热处理时间来精确控制材料的石墨化结构,系统地揭示了碳基材料的微观结构演变对竞争性VOC吸附行为的影响机制。当热处理时间延长到4 h时,得到的BFC800-4样品石墨化程度最高,其XPS数据中sp2-C / (sp2-C + sp3-C)的面积比增加到0.91。在苯/丙酮共吸附中,BFC800-4对苯的选择性较好(系数为5.57),对苯和丙酮的吸附量分别为5.29和0.95 mmol/g。多尺度模拟(GCMC/DFT)表明石墨化碳平面与苯之间的π-π相互作用增强了吸附。同时,氮氧官能团的引入破坏了碳表面电子分布,削弱了这些相互作用。因此,开发高石墨化、低官能团含量的多孔碳材料可以定向控制竞争吸附选择性。本研究的合成策略和建立的微观结构-吸附关系为苯- voc分离和推进功能碳设计提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tuning graphitic domains in porous carbon for enhanced competitive adsorption of benzene over acetone: the critical role of graphitization in separation selectivity
Porous activated carbon offers tunable surface chemistry, developed porosity, and environmental compatibility, with its high surface area providing abundant active sites for volatile organic compound (VOC) adsorption. In this study, benzimidazole served as the carbon precursor and potassium ferrate as the activating agent. We innovatively utilized heat treatment time to precisely control the graphitization structure of the material, systematically revealing the mechanism by which the microstructural evolution of carbon-based materials affects competitive VOC adsorption behavior. When heat treatment duration extended to 4 h, the resulting BFC800–4 sample exhibited the highest graphitization degree, and the area ratio of sp2-C / (sp2-C + sp3-C) in its XPS data increased to 0.91. In benzene/acetone co-adsorption, BFC800–4 showed exceptional benzene selectivity (coefficient: 5.57), with benzene and acetone adsorption capacities of 5.29 and 0.95 mmol/g, respectively. Multiscale simulations (GCMC/DFT) reveal enhanced adsorption stems from π-π interactions between graphitized carbon planes and benzene. Simultaneously, the introduction of nitrogen‑oxygen functional groups disrupts carbon surface electron distribution, weakening these interactions. Therefore, developing porous carbon materials with high graphitization and low functional group content enables directional control of competitive adsorption selectivity. The synthetic strategy and established microstructure-adsorption relationship in this study provide a theoretical basis for benzene-VOC separation and advance functional carbon design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


