细胞壁结合蛋白-武装控释纳米递送系统增强Nisin抗肺炎链球菌感染的疗效
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
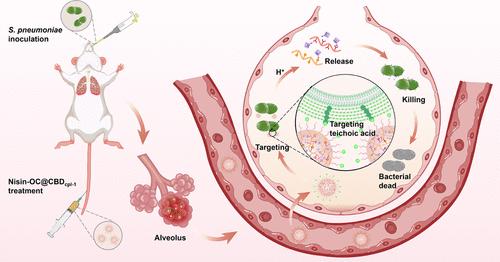
肺炎链球菌是一种主要的人类病原体,可导致危及生命的感染,特别是在全世界的儿童和老年人中。目前的预防和治疗战略,包括疫苗和抗生素,正日益受到非疫苗血清型的出现和抗生素耐药性上升的挑战。Nisin是一种针对脂质ii的肽抗生素,对肺炎链球菌有效,但生理pH值不稳定,需要创新的给药方法。在这里,我们开发了一种纳米递送系统,通过利用细菌感染的酸性微环境来提高nisin的稳定性和有效性。该系统利用氧化透明质酸和儿茶酚壳聚糖形成微环境响应型乳酸链球菌蛋白负载模块,并进一步功能化肺炎链球菌特异性内溶素细胞壁结合域(cbdpcl -1)进行靶向递送。该系统在模拟感染环境的酸性条件下表现出明显的感染位点积累和控制nisin释放。在耐抗生素肺炎链球菌诱导的肺炎小鼠模型中,与游离nisin相比,纳米递送系统显着提高了生存率并减少了细菌负荷,强调了其作为抗耐抗生素肺炎链球菌感染的强大工具的潜力。本研究为加强nisin和其他肽类抗生素的临床应用,解决耐药细菌病原体带来的紧迫挑战提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cell Wall-Binding Proteins-Armed Controlled-Release Nanodelivery System Enhances Nisin’s Efficacy against Streptococcus pneumoniae Infections
Streptococcus pneumoniae is a leading human pathogen responsible for life-threatening infections, particularly in children and the elderly worldwide. Current prevention and treatment strategies, including vaccines and antibiotics, are increasingly challenged by the emergence of nonvaccine serotypes and rising antibiotic resistance. Nisin, a lipid II-targeting peptide antibiotic, is effective against S. pneumoniae but suffers from instability at physiological pH, necessitating innovative delivery approaches. Here, we developed a nanodelivery system that enhances nisin’s stability and efficacy by exploiting the acidic microenvironment of bacterial infections. This system utilizes oxidized hyaluronic acid and catechol chitosan to form a microenvironment-responsive nisin-loading module, further functionalized with a S. pneumoniae-specific endolysin cell wall binding domain (CBDcpl-1) for targeted delivery. The system demonstrated significant infection site accumulation and controlled nisin release under acidic conditions, mimicking the infection environment. In a mouse model of antibiotic-resistant S. pneumoniae-induced pneumonia, the nanodelivery system significantly improved survival rates and reduced bacterial loads compared to free nisin, underscoring its potential as a powerful tool against antibiotic-resistant S. pneumoniae infections. This study presents a promising strategy for enhancing the clinical use of nisin and other peptide antibiotics, tackling the urgent challenge posed by resistant bacterial pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


