调整MoO3/V2O5纳米复合材料的Mo:V比以实现亚甲基蓝的高效表面吸附
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
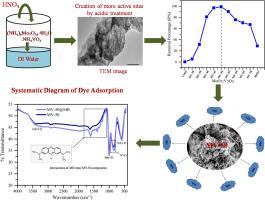
在这项研究中,使用一种简单、省时、经济的湿化学方法合成了MoO3/V2O5纳米复合材料(nc)。通过控制Mo:V比,制备了各种MoO₃/V₂O₅NCs (MV-30, MV-50, MV-70)。采用XRD、拉曼光谱、FESEM、EDS、HRTEM、zeta电位、beta - bjh、TGA和FTIR等分析手段对合成的纳米复合材料进行了表征。XRD谱图证实,随着Mo含量的增加,MoO₃峰强度逐渐增加。MV-50纳米材料的比表面积达到16.2 m²/g,平均孔径达到3.6 nm。合成的NC用于亚甲基蓝(MB)染料的吸附。MV-50纳米炭具有较高的比表面积和较大的孔径,对染料的吸附性能优于其他纳米炭。在初始染料浓度为20 mg/L、pH为中性、环境温度下,8分钟内99.9%的染料被吸附。动力学研究表明,MB的吸附符合准二级模型,表明其为化学吸附。平衡数据与Langmuir模型吻合良好,表明单层吸附的最大容量为737.6 mg/g。热力学参数表明,该反应为自发的吸热反应,固液界面处的吉布斯自由能为负8 kJ/mol,焓(∆H°)为6.58 kJ/mol,随机性增大(∆S°= 47.3 J/mol·K)。吸附后分析(包括TGA、FTIR、BET、EDS和FESEM)证实了MV-50的结构稳定性。它在含有干扰离子的工业废水样品中的可重复使用性和一致的性能突出了它在实际环境修复中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring the Mo:V Ratio in MoO3/V2O5 nanocomposites for high-efficiency surface adsorption of methylene blue
In this study, synthesis of MoO3/V2O5 nanocomposites (NCs) has been reported using a simple, time-efficient, and cost-effective wet chemical approach. Various MoO₃/V₂O₅ NCs (MV-30, MV-50, MV-70) have been prepared by controlling the Mo:V ratio. The synthesized nanocomposites were characterized using XRD, Raman spectroscopy, FESEM, EDS, HRTEM, zeta potential, BET-BJH, TGA, and FTIR analyses. XRD patterns confirmed a gradual increase in MoO₃ peak intensity with rising Mo content. The MV-50 NCs exhibited an enhanced specific surface area of 16.2 m²/g and an average pore size of 3.6 nm. The synthesized NC has been utilised for Methylene Blue (MB) dye adsorption. The MV-50 NCs show the best dye adsorption in comparison to other NCs, which is attributed to higher specific surface area and larger pore size. At an initial dye concentration of 20 mg/L, neutral pH, and ambient temperature, 99.9 % of the dye was adsorbed within 8 minutes. Kinetic studies revealed that MB adsorption followed the pseudo-second-order model, suggesting chemisorption. The equilibrium data aligned well with the Langmuir model, indicating monolayer adsorption with a maximum capacity of 737.6 mg/g. Thermodynamic parameters confirmed the process was spontaneous and endothermic, supported by negative Gibbs free energy (8 kJ/mol), an enthalpy (∆H°) of 6.58 kJ/mol, and increased randomness (∆S° = 47.3 J/mol·K) at the solid–liquid interface. Post-adsorption analyses, including TGA, FTIR, BET, EDS, and FESEM, demonstrated the structural stability of MV-50. Its reusability and consistent performance in industrial wastewater samples containing interfering ions highlight its potential for practical environmental remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


