碱性活化过硫酸盐在助溶剂辅助下对vocs污染土壤进行原位修复,提高修复效率
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
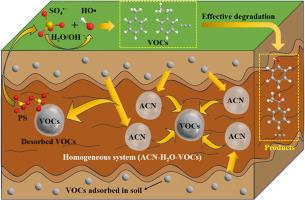
开发高效、可持续的土壤修复策略是治理挥发性有机化合物污染土壤的关键。研究了共溶剂辅助碱性活化过硫酸盐(PS)体系对污染土壤中甲基苯(MB)和乙苯(EB)的去除效果,并评价了共溶剂对MB和EB解吸和增溶的促进作用。在最佳条件(CPS = 382 mmol L−1,pH = 12, 40% (v/v)乙腈(ACN)为助溶剂)下,第1天对MB和EB的去除率分别达到41.5%和50.9%,显著高于无ACN的11.4%和16.2%。在第21天,对MB和EB的去除率分别达到91.7%和94.5%。乙基上较弱的C-C键(76.4 kcal mol−1)和C-H键(85.4 kcal mol−1)使EB具有较高的降解率。电子自旋共振(ESR)和淬火实验证实,•OH和SO4·−是主要的活性氧,对MB的降解作用分别为52.0%和35.2%。MB和EB的降解通过抽氢、羟基化、电子转移和脱烷基作用进行,最终实现完全矿化。利用T.E.S.T.和生物发光细菌试验进行的毒性评估表明,生态风险显著降低,对MB和EB的生物发光抑制率分别从70.7%(剧毒)和98.3%(剧毒)降至8.2%(低毒)和5.6%(低毒)。综上所述,本研究为vocs污染土壤的修复提供了一个有前景的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced in-situ remediation of VOCs-contaminated soil using alkaline activated persulfate with co-solvent assistance for improved removal efficiency
The development of efficient and sustainable remediation strategies is critical for treating volatile organic compound (VOCs)-contaminated soil. This study investigated the removal of methylbenzene (MB) and ethylbenzene (EB) from contaminated soil through co-solvent-assisted alkaline activation of persulfate (PS) system, The effect of the co-solvent in enhancing the desorption and solubilization of MB and EB was also evaluated. Under optimal conditions (CPS = 382 mmol L−1, pH 12, 40 % (v/v) acetonitrile (ACN) as co-solvent), the removal rates of MB and EB reached 41.5 % and 50.9 % on the 1st day, significantly higher than those without ACN (11.4 % and 16.2 %, respectively). Moreover, the removal rates further increased to 91.7 % for MB and 94.5 % for EB on the 21st day. The higher degradation rate of EB was attributed to the relatively weak benzylic C–C bond (76.4 kcal mol−1) and C–H bonds (85.4 kcal mol−1) at the ethyl site. Electron spin resonance (ESR) and quenching experiments confirmed that •OH and were the dominant reactive oxygen species, contributing 52.0 % and 35.2 % to MB degradation, respectively. The degradation of MB and EB proceeded via hydrogen abstraction, hydroxylation, electron transfer, and dealkylation, culminating in complete mineralization. Toxicity assessments using T.E.S.T. and bioluminescent bacteria assays indicated a significant reduction in ecological risk, with bioluminescence inhibition for MB and EB decreasing from 70.7 % (very toxic) and 98.3 % (very toxic) to 8.2 % (low toxicity) and 5.6 % (low toxicity), respectively. In summary, this study presents a promising strategy for the remediation of VOCs-contaminated soils.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


