功能化嘧啶类化合物的合成、表征、构效关系及对棉蚜的杀虫效果评价
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
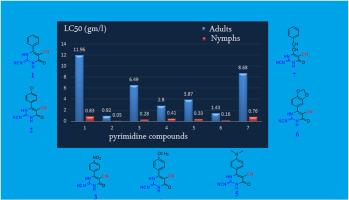
以氰胍、氰乙酸乙酯和多种芳香族醛为原料,通过一锅三组分反应合成了7种新型嘧啶衍生物。这些新合成的嘧啶化合物的化学结构通过元素分析和光谱分析得到了证实。所有化合物都具有NCN基团的特征,这是新烟碱类杀虫剂的一个特征。考虑到这一结构特征,我们选择了这些化合物来测试它们对影响棉花作物的主要害虫之一棉蚜(Aphis gossypii)的毒理学活性,棉蚜是造成棉花产量损失的主要原因。新烟碱类杀虫剂以其控制这种害虫的有效性而闻名。通过5种不同浓度的化合物对棉蚜成虫和若虫的杀虫活性进行了评价。实验结果表明,化合物2和6对棉蚜成虫和若虫均表现出最高的抑制活性。这些发现表明,这些化合物有可能作为新烟碱类杀虫剂的新类似物防治棉花蚜虫。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Functionalized pyrimidines: Synthesis, characterization, structure-activity relationship and insecticidal evaluation of some new neonicotinoid analogues against cotton aphid
Seven novel pyrimidine derivatives were synthesized through a one-pot three-component reaction involving cyanoguanidine, ethylcyanoacetate, and various aromatic aldehydes. The chemical structures of these newly synthesized pyrimidine compounds were confirmed by using both elemental and spectral analysis. All compounds feature the characteristic N![]() CN group, which is a defining trait of neonicotinoid insecticides. Given this structural feature, these compounds were selected for testing their toxicological activity against one of the major pests affecting cotton crops, Aphis gossypii, which is responsible for significant cotton yield loss. Neonicotinoid insecticides are known for their effectiveness in controlling this pest. Insecticidal activity was evaluated by testing five different concentrations of each compound against both adults and nymphs of A. gossypii. Laboratory results indicated that compounds 2 and 6 exhibited the highest levels of activity against both adults and nymphs of A. gossypii pest. These findings suggest that these compounds may offer potential as new analogues within the neonicotinoid insecticide class for controlling cotton aphids.
CN group, which is a defining trait of neonicotinoid insecticides. Given this structural feature, these compounds were selected for testing their toxicological activity against one of the major pests affecting cotton crops, Aphis gossypii, which is responsible for significant cotton yield loss. Neonicotinoid insecticides are known for their effectiveness in controlling this pest. Insecticidal activity was evaluated by testing five different concentrations of each compound against both adults and nymphs of A. gossypii. Laboratory results indicated that compounds 2 and 6 exhibited the highest levels of activity against both adults and nymphs of A. gossypii pest. These findings suggest that these compounds may offer potential as new analogues within the neonicotinoid insecticide class for controlling cotton aphids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


