使用生物正交点击交联明胶水凝胶进行体外淋巴管生成的三维建模
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
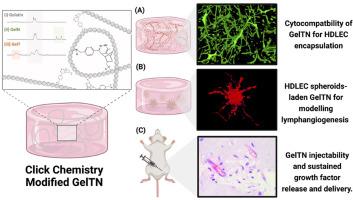
淋巴管生成,即由原有淋巴管形成新的淋巴管,对于维持组织稳态和免疫功能至关重要。尽管最近在理解调节淋巴管生成的分子机制方面取得了进展,但大多数体外研究依赖于传统的二维(2D)细胞培养,对体内控制淋巴管生成的复杂微环境的复制有限。在这里,我们提出了一个三维(3D)淋巴管生成模型,使用明胶水凝胶修饰点击化学基元(四嗪和降冰片烯,GelTN),为淋巴内皮细胞提供仿生和机械可调的细胞外基质(ECM)。通过将人真皮淋巴内皮细胞(HDLEC)球体包埋在GelTN中,我们建立了一个强大而可靠的体外发芽实验(48小时持续时间),以研究GelTN硬度对淋巴管生成的影响。与高GelTN包埋的HDLEC相比,低GelTN包埋的HDLEC在血管内皮生长因子(VEGF)-C刺激下发芽增强。我们还提供了β3整合素参与淋巴管生成的证据。抑制α5β1后,经β3整合素抑制后的芽长减少进一步减少,提示整合素亚基在控制HDLEC-ECM机械转导过程中存在协同作用。GelTN水凝胶也被评估了其翻译潜力,证明了VEGF-C在体外的持续释放,并在体内小鼠模型中皮下注射后支持细胞浸润和新血管形成。总的来说,这些发现突出了GelTN水凝胶作为研究淋巴管生成的平台的多功能性,以及它们在组织工程和再生医学中需要控制生长因子输送的治疗应用的潜在用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Three-dimensional modelling of lymphangiogenesis in-vitro using bioorthogonal click-crosslinked gelatin hydrogels
Lymphangiogenesis, the formation of new lymphatic vessels from pre-existing vessels, is crucial for maintaining tissue homeostasis and immune function. Despite recent advances in understanding the molecular mechanisms regulating lymphangiogenesis, most in vitro studies rely on traditional two-dimensional (2D) cell cultures, with limited replication of the complex microenvironment that governs lymphangiogenesis in vivo. Here, we present a three-dimensional (3D) lymphangiogenesis model using gelatin hydrogels modified with click-chemistry motifs (tetrazine and norbornene, GelTN), providing a biomimetic and mechanically tunable extracellular matrix (ECM) for lymphatic endothelial cells. By encapsulating human dermal lymphatic endothelial cells (HDLEC) spheroids in GelTN, we established a robust and reliable in vitro sprouting assay (<48 h duration) to investigate the effects of GelTN stiffness on lymphangiogenesis. HDLEC encapsulated in low GelTN concentrations exhibited enhanced sprouting in response to vascular endothelial growth factor (VEGF)-C stimulation, compared to HDLEC encapsulated in higher GelTN concentrations. We also provide evidence for the involvement of β3 integrin in lymphangiogenesis. The reduced sprout length upon β3 integrin inhibition further decreased with combined inhibition of α5β1, suggesting a synergistic interaction of the integrin subunits in controlling HDLEC-ECM mechanotransduction. GelTN hydrogels were also evaluated for their translational potential, demonstrating sustained release of VEGF-C in vitro and supporting cellular infiltration and neo-vessel formation following subcutaneous injection in an in vivo mouse model. Overall, these findings highlight the versatility of GelTN hydrogels as a platform for studying lymphangiogenesis and their potential use for therapeutic applications that require controlled growth factor delivery in tissue engineering and regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


