用于高温CO2捕获的CaO/SiC(O)纳米复合材料:合成、表征和初始性能研究
IF 2.8
Q1 MATERIALS SCIENCE, CERAMICS
引用次数: 0
摘要
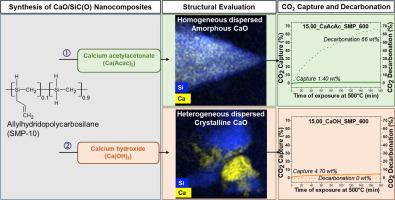
采用乙酰丙酮钙(CaAcac)和氢氧化钙(CaOH)两种钙前驱体对烯丙基多聚碳硅烷(SMP)进行化学改性,制备了CaO/SiC(O)纳米复合材料。ATR-FTIR分析表明,CaAcac与SMP发生化学相互作用,促进硅氢化和广泛交联,而CaOH则没有相互作用。热重分析表明,caacac修饰的SMP样品的陶瓷产率高于预期,而caoh修饰的SMP样品符合理论值。XRD和TEM结果表明,在600℃下,CaAcac主要生成非晶陶瓷,而CaOH则促进了CaO的结晶。纳米复合材料在500°C下捕获2-5 wt%的CO2,由于其低CaO含量(11 - 15%),这与CaCO3衍生的纯CaO的吸收率相当。然而,只有无定形的CaAcac修饰的纳米复合材料才能在500℃下释放吸收的CO2,释放量为66 wt%。因此,与caoh修饰的纳米复合材料相比,caacac修饰的SMP纳米复合材料在500°C的中等温度下具有更高的二氧化碳捕获和释放应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CaO/SiC(O) nanocomposites for CO2 capture at higher temperatures: Synthesis, characterization, and initial performance studies
This study presents the synthesis and characterization of CaO/SiC(O) nanocomposites via chemical modification of allylhydridopolycarbosilane (SMP) using two calcium precursors: calcium acetylacetonate (CaAcac) and calcium hydroxide (CaOH). ATR-FTIR analysis showed that CaAcac chemically interacts with SMP, promoting hydrosilylation and extensive cross-linking, while CaOH showed no interaction. Thermogravimetric analysis revealed higher than expected ceramic yields for CaAcac-modified SMP samples, whereas CaOH-modified SMP samples matched theoretical values. XRD and TEM results showed that CaAcac leads to mostly amorphous ceramics, while CaOH facilitated CaO crystallization at 600 °C. The nanocomposites captured 2–5 wt% CO2 at 500 °C, which is comparable to the uptake of pure CaO derived from CaCO3 due to their low CaO content (11–15 %). However, only the amorphous CaAcac modified nanocomposite can release the absorbed CO2 at 500 °C in larger amounts of 66 wt%. CaAcac-modified SMP nanocomposites thus show a higher potential for CO2 capture and release applications at moderate temperatures of 500 °C compared to CaOH-modified nanocomposites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Open Ceramics
Materials Science-Materials Chemistry
CiteScore
4.20
自引率
0.00%
发文量
102
审稿时长
67 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


