一种具有ros反应的纳米载体共递送铈纳米酶和沙利度胺用于协同治疗溃疡性结肠炎
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
溃疡性结肠炎(UC)是一种慢性和复发性胃肠道炎症性疾病,由于其发病率增加和复杂的发病机制涉及免疫失调和氧化应激,给临床带来了重大挑战。目前的治疗方法往往受到疗效差和严重副作用的限制,迫切需要改进治疗方法。在这里,我们提出了一种新的ROS反应性纳米载体(TPN),通过清除ROS和免疫调节来协同治疗结肠炎。将氧化铈纳米酶(Ceria NPs)和沙利度胺(Tha)包封在一种特殊设计的两亲性ros响应聚合物(PP)中制备TPN。TPN具有较强的活性氧清除活性,并能触发活性氧释放。在体外,TPN可有效减轻ros诱导的氧化应激和细胞凋亡。在dss诱导的小鼠结肠炎模型中,TPN在结肠内被动积累,显著减轻体重减轻、结肠缩短、疾病活动指数和组织学损伤。从机制上讲,TPN调节免疫反应,减少氧化应激,重塑肠道微生物群,使其更健康。此外,生物安全评估证实不存在全身毒性。总的来说,这些发现表明TPN通过整合靶向递送与协同抗氧化和免疫调节功能,代表了UC的有效和安全的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
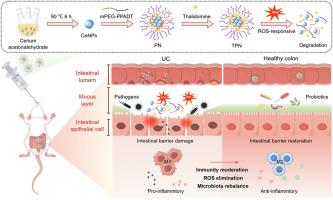
A ROS-responsive nanocarrier co-delivering cerium nanozymes and thalidomide for synergistic ulcerative colitis therapy
Ulcerative colitis (UC) is a chronic and recurrent inflammatory disorder of the gastrointestinal tract, posing major clinical challenges due to its increasing incidence and complex pathogenesis involving immune dysregulation and oxidative stress. Current therapies are often limited by poor efficacy and severe side effects, leaving an urgent need for improved treatments. Here, we present a novel ROS-responsive nanocarrier (TPN) for the synergistic therapy of colitis via ROS scavenging and immune modulation. TPN were fabricated by encapsulating cerium oxide nanozymes (Ceria NPs) and thalidomide (Tha) within a specially designed amphiphilic ROS-responsive polymer (PP). TPN exhibited strong ROS-scavenging activity and ROS-triggered Tha release. In vitro, TPN effectively attenuated ROS-induced oxidative stress and apoptosis. In a DSS-induced mouse colitis model, TPN passively accumulated in the colon and significantly alleviated body weight loss, colon shortening, disease activity index, and histological damage. Mechanistically, TPN modulated the immune response, reduced oxidative stress, and reshaped the gut microbiota toward a healthier composition. Moreover, biosafety assessments confirmed the absence of systemic toxicity. Collectively, these findings demonstrate that TPN represents a potent and safe therapeutic strategy for UC by integrating targeted delivery with synergistic antioxidant and immunomodulatory functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


