用于原位肿瘤疫苗的电激活纳米酶:协同多酶催化和电动力治疗
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
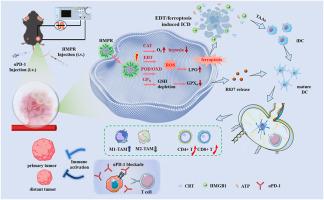
口腔鳞状细胞癌(OSCC)是一种高度侵袭性的恶性肿瘤,预后差,治疗方案有限。为了解决常规治疗耐药、抗氧化防御和免疫逃避的挑战,我们开发了一种多功能纳米酶平台MIL-100(Fe)@Pt@R837@ ha (HMPR),该平台集成了电动力治疗(EDT)、化学动力治疗和免疫治疗。HMPR的多酶活性允许氧气生成,羟基自由基产生和谷胱甘肽消耗,从而减轻缺氧,引发细胞凋亡和铁凋亡。在电刺激下,HMPR通过pt介导的EDT促进活性氧的显著产生,从而放大铁凋亡和免疫原性细胞死亡(ICD)。此外,肿瘤特异性GSH水平触发免疫佐剂R837的释放。强大的ICD效应,结合佐剂R837的持续释放,使纳米酶HMPR具有原位“肿瘤疫苗”的特性,持续促进树突状细胞成熟,激活T细胞介导的免疫,建立长期免疫记忆,以防止肿瘤复发和抑制肿瘤远处生长。这种级联效应将免疫抑制的肿瘤微环境重编程为免疫刺激的微环境,显著增强了PD-1阻断的功效。我们的研究为OSCC提供了一个强大的电增强纳米酶免疫协同策略,并提供了一个灵活的平台,适用于其他实体肿瘤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electro-activated nanozyme for in situ tumor vaccination: Synergistic multi-enzyme catalysis and electrodynamic therapy
Oral squamous cell carcinoma (OSCC) is a highly aggressive malignancy with poor prognosis and limited treatment options. To address the challenges of conventional therapy resistance, antioxidant defence, and immune evasion, we developed a multifunctional nanozyme platform, MIL-100(Fe)@Pt@R837@HA (HMPR), that integrates electrodynamic therapy (EDT), chemodynamic therapy, and immunotherapy. The multi-enzyme activities of HMPR allow for the oxygen generation, hydroxyl radicals production, and glutathione depletion, thereby alleviating hypoxia and triggering both apoptosis and ferroptosis. Under electrical stimulation, HMPR promotes significant production of reactive oxygen species via Pt-mediated EDT to amplify ferroptosis and immunogenic cell death (ICD). In addition, the tumor-specific GSH level triggers the release of the immune adjuvant R837. The potent ICD effect, combined with the sustained release of the adjuvant R837, endows the nanozyme HMPR with the characteristics of an in situ “tumor vaccine”, continuously promoting dendritic cell maturation, activating T cell-mediated immunity, and establishing long-term immunological memory to prevent tumor recurrence and inhibition of distant tumor growth. This cascade effect reprograms the immunosuppressive tumor microenvironment into an immunostimulatory one, significantly enhancing the efficacy of PD-1 blockade. Our study provides a powerful electro-enhanced nanozyme-immune synergistic strategy for OSCC and offers a flexible platform adaptable to other solid tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


