磁性水凝胶在肝细胞癌磁热治疗中诱导多程序细胞死亡和肿瘤深度消退
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
肝细胞癌因其复杂的发病机制和缺乏有效的治疗药物,一直是全球关注的健康问题。近年来,在癌症治疗新技术的探索中,磁热疗法(MHT)成为一种有前途和安全的治疗策略。在此,我们首次报道了一种可注射的、基于磁水凝胶结构的热回归剂mPEG-b-PLV-Fe3O4杂种在MHT中的应用。经皮热疗是一种微创治疗兔肝脏肿瘤的方法。在体外和体内实验的基础上,我们证明了mPEG-b-PLV-Fe3O4杂种在磁场作用下,通过诱导大量ROS积累、严重脂质过氧化和线粒体损伤,从而引发细胞死亡的三种形式:铁凋亡、自噬和凋亡,表现出强大的抗肿瘤作用。此外,我们发现mPEG-b-PLV-Fe3O4杂种在磁场作用下通过抑制SLC7A11表达重编程半胱氨酸-谷胱甘肽代谢,从而加剧氧化还原失衡,抑制细胞存活。该杂交体在小鼠和家兔模型中取得了显著的治疗效果。我们的研究结果强调,磁场下mPEG-b-PLV-Fe3O4杂种通过靶向slc7a11 -半胱氨酸-谷胱甘肽轴,是一种很有前景的癌症治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
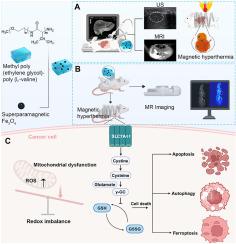
Injectable magnetic hydrogel induces multi-programmed cell death and deep tumor regression in magnetic hyperthermia therapy in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) remains global health concern due to complex pathogenic mechanism and lack of effective therapeutic drugs. The magnetic hyperthermia therapy (MHT) appears as the promising and safe strategy during the recent exploration of new technology for cancer treatment. Herein, we reported an injectable and magnetic-hydrogel-construct-based thermal regression agent mPEG-b-PLV-Fe3O4 Hybrids in MHT for the first time. Percutaneous thermotherapy was employed as a minimally invasive strategy for treating hepatic tumors in a rabbit model. Based on the evidence in vitro and in vivo studies, we demonstrated that mPEG-b-PLV-Fe3O4 Hybrids under magnetic field, exhibited powerful antitumor effect by inducing massive ROS accumulation, severe lipid peroxidation and damaged mitochondria, which together triggered triple forms of cell death: ferroptosis, autophagy and apoptosis. Furthermore, we revealed that mPEG-b-PLV-Fe3O4 Hybrids under magnetic field reprogramed the cysteine-glutathione metabolism by suppressing SLC7A11 expression, which aggravated redox imbalance and suppresses cell survival. The hybrids achieve outstanding therapeutic results in mouse and rabbit models. Our findings underscore that mPEG-b-PLV-Fe3O4 Hybrids under magnetic field is a promising strategy for cancer therapy by targeting the SLC7A11-cysteine-glutathione axis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


