通过3D支架微结构工程巨噬细胞反应
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
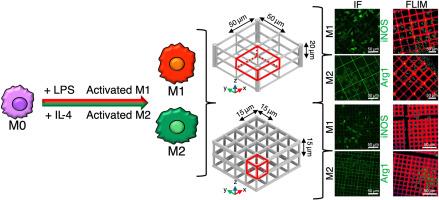
在活体组织中植入生物材料会引发一种被称为异物反应的生理反应,导致巨噬细胞的募集,巨噬细胞可以分化为促炎(M1)或抗炎(M2)表型。目前,人们对定制生物材料的物理特性以促进有效的组织再生越来越感兴趣。三维结构可以深刻地影响巨噬细胞的行为;然而,对潜在的机制没有明确的共识。三维微结构可能在调节免疫细胞、促进抗炎反应和支持有效的组织修复和再生方面发挥关键作用。本研究采用双光子聚合的方法设计并制备了大孔(50 × 50 × 20 μm3)和小孔(15 × 15 × 15 μm3)的三维支架。这两种微观结构都有效地影响巨噬细胞的细胞骨架组织和细胞代谢活性。值得注意的是,它们不足以诱导巨噬细胞自发极化,这表明它们本质上是免疫惰性的。当与化学刺激相结合时,就像生理上通常发生的那样,它们引发了不同的反应。通过对两种孔径的研究,我们发现了抗炎和促炎表型之间的平衡,大孔径的Arg1表达轻微上调,小孔径的iNOS表达明显增加。我们的研究结果表明,3D微结构是多种应用的通用工具。它们精确可调的结构能够精细控制巨噬细胞的行为,通过防止纤维化和促进抗炎和促再生反应,为体内组织工程开辟了新的途径,并为开发体外平台来模拟炎症组织以筛选抗炎药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineering macrophage responses through 3D scaffold microarchitecture
Biomaterial implantation in living tissues triggers a physiological response known as foreign body reaction, leading to the recruitment of macrophages, that can polarize either into a pro-inflammatory (M1) or an anti-inflammatory (M2) phenotype. Currently, there is growing interest in tailoring the physical properties of biomaterials to promote efficient tissue regeneration. Tridimensionality can profoundly influence macrophage behaviour; however, there is no clear consensus on the underlying mechanisms. 3D microstructures may play a crucial role in modulating immune cells, promoting anti-inflammatory responses, and supporting effective tissue repair and regeneration. In this study, we designed and fabricated 3D scaffolds with large pores (50 × 50 × 20 μm3) and small pores (15 × 15 × 15 μm3) by two-photon polymerization. Both microstructures effectively influenced macrophage cytoskeletal organization and cellular metabolic activity. Notably, they were not sufficient to induce spontaneous macrophage polarization, indicating that they are intrinsically immunologically inert. When combined with chemical stimulation, as typically occurs physiologically, they elicited distinct responses. The investigation of two pore sizes allowed us to find a balance between the anti-inflammatory and pro-inflammatory phenotypes, with a slight upregulation of Arg1 by large pores, and a marked increase of iNOS expression by small pores. Our results demonstrate that 3D microstructures are versatile tools for multiple applications. Their precisely tunable architecture enables fine control over macrophage behaviour, opening new avenues both for in vivo tissue engineering, by preventing fibrosis and promoting anti-inflammatory and pro-regenerative responses, and for the development of in vitro platforms to model inflamed tissues for screening anti-inflammatory drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


