光触发的一氧化碳诱导的强化铁蛋白吞噬介导的铁凋亡的激活用于骨转移治疗
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨转移是各种实体瘤晚期常见的致残和危及生命的并发症,由于其高药物毒性和肿瘤耐药性,继续给治疗带来巨大挑战。为了克服现有治疗方法有限的疗效和安全性问题,我们开发了一种新的铁基光催化纳米平台(ENCF),由第二次近红外(NIR-II)成像引导,用于骨转移的精确治疗。该平台可以实现CO的原位光催化释放,并利用暴露的铁活性位点在808 nm激光激活下通过氧化应激、自噬和铁代谢破坏级联协同诱导铁死亡。机制研究表明,ENCF平台显著下调铁蛋白自噬的关键调控因子PCBP2,同时激活LC3-和atg5介导的自噬途径,加速FTH1降解和Fe2+释放,从而扰乱细胞内铁稳态。同时,释放的CO破坏线粒体电子传递,抑制ATP合成,导致ROS过度积累,增强GPX4的抑制,加速脂质过氧化,并启动强大的铁致凋亡反应。得益于其深层组织光激活、高催化效率和多靶点协同机制,ENCF在骨转移模型中实现了有效的肿瘤抑制,并在转移部位选择性积累。总的来说,本研究建立了一个多管齐下的治疗策略,通过“CO释放-自噬增强-凋亡激活”,为骨转移的精确治疗提供了一个有前途的创新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
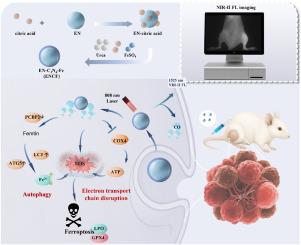
Light-triggered carbon monoxide-induced activation of enhanced ferritinophagy-mediated ferroptosis for bone metastases therapy
Bone metastases, as a common disabling and life-threatening complication in the advanced stages of various solid tumors, continue to pose substantial therapeutic challenges due to high drug toxicity and tumor resistance. To overcome the limited efficacy and safety concerns of existing treatments, we developed a novel iron-based photocatalytic nanoplatform (ENCF), guided by second near-infrared (NIR-II) imaging, for the precise treatment of bone metastases. This platform enables in situ photocatalytic release of CO and utilizes exposed iron active sites to synergistically induce ferroptosis through a cascade of oxidative stress, autophagy and iron metabolism disruption under 808 nm laser activation. Mechanistic investigations revealed that the ENCF platform significantly downregulates PCBP2, a key regulator of ferritinophagy, while activating LC3- and ATG5-mediated autophagic pathways to accelerate FTH1 degradation and Fe2+ release, thereby disturbing intracellular iron homeostasis. Concurrently, the released CO disrupts mitochondrial electron transport and inhibits ATP synthesis, leading to excessive ROS accumulation, enhanced suppression of GPX4 , accelerated lipid peroxidation, and the initiation of a robust ferroptotic response. Benefiting from its deep-tissue photoactivation, high catalytic efficiency, and multi-target synergistic mechanisms, ENCF achieved potent tumor suppression with selective accumulation at metastatic sites in a bone metastasis model. Collectively, this study establishes a multi-pronged therapeutic strategy via “CO release–autophagy enhancement–ferroptosis activation,” offering a promising and innovative approach for the precise treatment of bone metastases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


