家蚕动力蛋白基因硒协同RNAi的组织学和分子分析
IF 1.3
3区 农林科学
Q3 ENTOMOLOGY
引用次数: 0
摘要
硒(Se)是动物体内必需的元素,调节多种生化过程和生长发育。我们之前的研究表明,50 μM Se对家蚕(Bombyx mori) 24 h内噬作用的关键蛋白动力蛋白(dynamin)产生了显著影响。本研究探讨了不同处理下家蚕血淋巴代谢物水平和蚕丝腺基因表达的变化。非靶向代谢组学分析显示,动力蛋白敲低组有297种差异代谢物(80种上调,217种下调),硒添加组有295种差异代谢物(204种上调,91种下调),包括氨基酸、糖、脂质、核苷酸代谢物及其衍生物。基因敲低增强了天冬氨酸的生物合成,但减弱了糖酵解/糖异生、TCA循环、嘌呤代谢、丙氨酸和谷氨酸代谢途径。相反,硒显著增强糖酵解/糖异生、TCA循环、嘌呤代谢和丙氨酸/天冬氨酸/谷氨酸途径,同时抑制赖氨酸的生物合成。对丝腺转录组学分析发现,敲除组有705个差异表达基因(deg),向上表达320个,向下表达385个;Se组有2907个差异表达基因(deg),向上表达2714个,向下表达193个。基因敲低影响蛋白加工、MAPK信号传导和内吞作用,而硒影响谷胱甘肽代谢、剪接体和过氧化物酶体途径。这些deg与蛋白质加工、能量供应和抗氧化剂有关。本研究阐明了硒相关RNAi应激(动力蛋白敲低)对家蚕代谢和转录的影响,强调了它们在关键生理过程中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
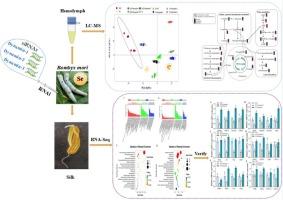
Histological and molecular analysis of selenium synergistic RNAi of Dynamin gene in Bombyx mori
Selenium (Se) is an essential element for animals, regulating various biochemical processes as well as growth and development. Our previous study showed that 50 μM Se significantly affected the silkworm, Bombyx mori by targeting dynamin, a key endocytosis protein, at 24 h. Here, we explored the hemolymph metabolite levels and silk gland gene expression in B. mori under different treatments. Non-targeted metabolomics analyses revealed 297 differential metabolites (80 up-regulated and 217 down-regulated) in the dynamin knockdown group and 295(204 up and 91 down) in the Se additive group, including amino acids, sugars, lipids, nucleotide metabolites, and their derivatives. The knockdown enhanced aspartate biosynthetic but weakened glycolysis/gluconeogenesis, the TCA cycle, purine metabolism, alanine and glutamate metabolic pathways. In contrast, Se significantly enhanced glycolysis/gluconeogenesis, the TCA cycle, purine metabolism, and alanine,/aspartate/ glutamate pathways while inhibiting lysine biosynthesis. Transcriptomic analysis of the silk gland identified 705 differentially expressed genes (DEGs) (320 up and 385 down) in the knockdown group and 2907 DEGs (2714 up and 193 down) in the Se group. Knockdown affected protein processing, MAPK signaling, and endocytosis, while Se impacted glutathione metabolism, spliceosome, and peroxisomal pathways. These DEGs are linked to protein processing, energy provision, and antioxidants. This study elucidates the metabolic and transcriptional changes due to Se-associated RNAi stress (dynamin knockdown) on silkworms, highlighting their roles in key physiological processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Asia-pacific Entomology
Agricultural and Biological Sciences-Insect Science
CiteScore
2.70
自引率
6.70%
发文量
152
审稿时长
69 days
期刊介绍:
The journal publishes original research papers, review articles and short communications in the basic and applied area concerning insects, mites or other arthropods and nematodes of economic importance in agriculture, forestry, industry, human and animal health, and natural resource and environment management, and is the official journal of the Korean Society of Applied Entomology and the Taiwan Entomological Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


