基于3D打印聚羟基烷酸酯支架的脂肪移植
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-20
DOI:10.1016/j.bioadv.2025.214512
引用次数: 0
摘要
自体脂肪移植在软组织修复和重建方面具有重要的前景。然而,其临床应用面临挑战,包括移植物强度不足,无法达到最佳塑形,长期保留率差,特别是在大容量移植中。三维(3D)打印的可生物降解支架提供了一种潜在的解决方案,通过减轻移植物缺血和缺氧,从而提高保留率,同时在降解前提供暂时的机械支持。本研究采用含有3-羟基丁酸酯和4-羟基丁酸酯单体的聚羟基烷酸酯(PHA)作为生物材料,制备了脂肪移植3d打印支架。探讨了它们对移植物滞留的影响的潜在机制。体内研究表明,3d打印的PHA支架通过刺激血管生成、促进脂肪细胞活力、诱导巨噬细胞向M2表型极化、减轻氧化应激和优化线粒体功能,显著增强了脂肪移植保留。此外,支架通过促进米色脂肪生成或白色脂肪组织褐变进一步改善滞留。体外实验证实PHA具有良好的生物相容性,其降解产物- 3-羟基丁酸酯(3HB)无细胞毒性。此外,3HB通过减少氧化应激和改善线粒体功能,增强了脂肪源性干细胞(ADSCs)的能量代谢。本研究为PHA支架在脂肪组织工程中的应用提供了有力的证据,并为软组织重建和修复提供了一种新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
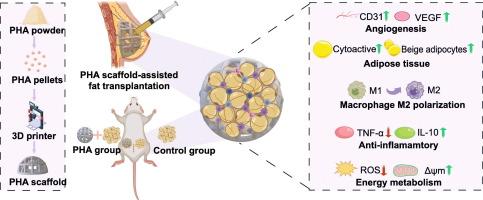
Fat grafting based on 3D printed polyhydroxyalkanoate scaffolds
Autologous fat grafting holds significant promise for soft tissue repair and reconstruction. However, its clinical application faces challenges, including insufficient graft strength for optimal shaping and poor long-term retention rates, particularly in large-volume transplantation. Three-dimensional (3D)-printed biodegradable scaffolds offer a potential solution by mitigating graft ischemia and hypoxia, thereby improving retention, while offering temporary mechanical support before degradation. Polyhydroxyalkanoates (PHA) containing 3-hydroxybutyrate and 4-hydroxybutyrate monomers, a promising class of biomaterials in tissue engineering, were employed in this study to fabricate 3D-printed scaffolds for fat grafting. Their effects on graft retention were explored for underlying mechanisms. In vivo studies demonstrated that 3D-printed PHA scaffolds significantly enhanced fat graft retention by stimulating angiogenesis, promoting adipocyte viability, inducing macrophage polarization toward the M2 phenotype, attenuating oxidative stress, and optimizing mitochondrial functions. Additionally, the scaffolds further improved retention by facilitating beige adipogenesis or white adipose tissue browning. In vitro experiments confirmed the excellent biocompatibility of PHA, with its degradation product ‐3-hydroxybutyrate (3HB) exhibiting no cytotoxicity. Furthermore, 3HB enhanced the energy metabolism of adipose-derived stem cells (ADSCs) by reducing oxidative stress and improving mitochondrial function. This study provides solid evidence supporting the application of PHA scaffolds in adipose tissue engineering and proposes a novel strategy for soft tissue reconstruction and repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


