gsh触发CD44/FRβ双靶向纳米前药通过级联DNA损伤和线粒体氧化风暴根除急性髓系白血病
IF 4.9
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
传统的DNA双链断裂(DSBs)诱导的急性髓性白血病(AML)化疗药物通常受到溶解度差、非选择性和来自强大的肿瘤DNA修复机制的耐药性的限制。在这里,我们报告谷胱甘肽(GSH)反应,双靶向纳米前药,指定HA-FA@Etp-Olp,用于有效的AML细胞根除。通过二硫键将PARP抑制剂Olaparib (Olp)与依托泊苷(etoposide)偶联,构建了HA-FA@Etp-Olp体系,形成Etp-Olp异二聚体前药。这种疏水共轭物被封装在由聚乙二醇修饰透明质酸(HA)和叶酸(FA)组成的聚合物载体中,自组装成精细的纳米颗粒。HA-FA@Etp-Olp表现出优异的循环稳定性,利用CD44/FR双受体介导的活性靶向,在AML细胞内实现了高效的积累,随后是GSH触发的分解和特异性药物释放,以响应细胞内GSH水平的升高。此外,HA-FA@Etp-Olp通过双管齐下的机制引发了抗AML的协同细胞毒性作用:“DNA损伤-修复阻断”级联和线粒体氧化应激的显著增强,有效诱导凋亡细胞死亡。该策略提供了一种有前景的靶向纳米治疗方法,具有增强的疗效和降低的全身毒性,显示出精确治疗AML的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
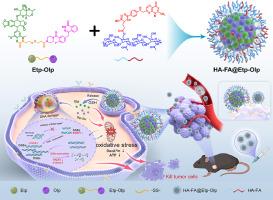
GSH-triggered CD44/FRβ dual-targeting nanoprodrug for acute myeloid leukemia eradication via cascade DNA damage and mitochondrial oxidative storm
Conventional DNA double-strand breaks (DSBs)-inducing chemotherapeutics for acute myeloid leukemia (AML) are often limited by poor solubility, non-selectivity, and drug resistance stemming from robust tumor DNA repair mechanisms. Here, we report a glutathione (GSH)-responsive, dual-targeting nano-prodrug, designated HA-FA@Etp-Olp, for efficient AML cell eradication. The HA-FA@Etp-Olp system was constructed through the conjugation of the PARP inhibitor Olaparib (Olp) with etoposide (Etoposide) via a disulfide linkage, forming an Etp-Olp heterodimeric prodrug. This hydrophobic conjugate was encapsulated within a polymeric carrier composed of poly (ethylene glycol)-modified hyaluronic acid (HA) functionalized with folic acid (FA), self-assembling into well-refined nanoparticles. Exhibiting excellent circulatory stability, HA-FA@Etp-Olp achieved efficient accumulation within AML cells leveraging CD44/FR dual-receptor-mediated active targeting, followed by GSH-triggered disassembly and specific drug release in response to elevated intracellular GSH levels. Furthermore, HA-FA@Etp-Olp elicited a synergistic cytotoxic effect against AML through a dual-pronged mechanism: "DNA damage-repair blockade" cascade and significant augmentation of mitochondrial oxidative stress, effectively inducing apoptotic cell death. This strategy provides a promising targeted nanotherapeutic approach with enhanced efficacy and reduced systemic toxicity, demonstrating significant potential for the precise treatment of AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

GIANT
Multiple-
CiteScore
8.50
自引率
8.60%
发文量
46
审稿时长
42 days
期刊介绍:
Giant is an interdisciplinary title focusing on fundamental and applied macromolecular science spanning all chemistry, physics, biology, and materials aspects of the field in the broadest sense. Key areas covered include macromolecular chemistry, supramolecular assembly, multiscale and multifunctional materials, organic-inorganic hybrid materials, biophysics, biomimetics and surface science. Core topics range from developments in synthesis, characterisation and assembly towards creating uniformly sized precision macromolecules with tailored properties, to the design and assembly of nanostructured materials in multiple dimensions, and further to the study of smart or living designer materials with tuneable multiscale properties.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


