用于治疗胶质母细胞瘤的新型鼻内原位热可逆性酮洛芬纳米凝胶的开发和表征
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-27
DOI:10.1016/j.jddst.2025.107580
引用次数: 0
摘要
酮洛芬(KET)已被证明对胶质母细胞瘤有效。然而,它的疏水性和给药后易降解,再加上血脑屏障的低渗透性,使其重新用于脑癌治疗具有挑战性。考虑到这一点,本研究的目的是开发ket负载的原位热可逆纳米凝胶,用于经鼻至脑给药,旨在增加药物强度和保护,控制药物释放,提高生物利用度。通过自发乳化制得含有Capryol®90、Tween®80、Transcutol®HP、poloxamer 407和水的配方。对制剂的滴度、PDI、zeta电位、pH、流变性、稳定性、体外释药、体外抗肿瘤疗效和安全性进行了评价。获得了高药物强度(4 mg/mL),小(20-30 nm)和单分散(PDI 0.1-0.2)纳米液滴,具有略负至中性的zeta电位(- 1.5至- 10 mV)。纳米凝胶的皮肤pH值(≈6)、鼻腔温度(32°C)、黏度(13660-927302 cP)升高、体外药物累积控释率(≈78 - 93%)符合Makoid-Banakar和Weibull动力学模型。优化后的纳米凝胶对人胶质瘤U87细胞有一定的抑制作用。因此,鼻内热可逆性ket负载纳米凝胶被成功开发,具有创新的成分,在胶质母细胞瘤治疗中显示出有希望的结果。考虑到它们的高可扩展性潜力,所开发的纳米平台可能是转化应用的有希望的候选者,未来的体内试验可以进一步证实它们的潜力,因此它们可能有一天被认为是胶质母细胞瘤的辅助或主要治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
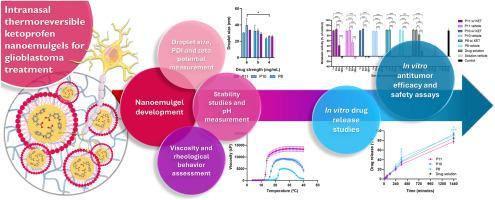
Development and characterization of novel intranasal in situ thermoreversible ketoprofen-loaded nanoemulgels for the treatment of glioblastoma
Ketoprofen (KET) has been proven effective against glioblastoma. Nevertheless, its hydrophobic nature and susceptibility to degradation upon administration, added to the blood-brain barrier's low permeability, make its repurposing for brain cancer treatment challenging. Having this in mind, the purpose of this work was to develop KET-loaded in situ thermoreversible nanoemulgels for intranasal nose-to-brain delivery, aiming at increased drug strength and protection, controlled drug release, and improved bioavailability. Formulations containing Capryol® 90, Tween® 80, Transcutol® HP, poloxamer 407, and water were produced through spontaneous emulsification. Formulations' droplet size, polydispersity index (PDI), zeta potential, pH, rheology, stability, in vitro drug release, and in vitro antitumor efficacy and safety were evaluated. A high drug strength (4 mg/mL), and small (20–30 nm) and monodisperse (PDI 0.1–0.2) nanodroplets, with slightly negative to neutral zeta potential (−1.5 to −10 mV), were obtained. The nanoemulgels' also revealed skin adequate pH (≈6), nasal cavity temperature’ sol-gel transitions (32 °C), elevated viscosity (13660–927302 cP), and high cumulative controlled in vitro drug release (≈78–93 %), following Makoid-Banakar and Weibull kinetic models. Optimized nanoemulgels revealed relevant efficacy against human glioblastoma U87 cells. Therefore, intranasal thermoreversible KET-loaded nanoemulgels were successfully developed, of innovative composition, showing promising results for glioblastoma treatment. Given their high scalability potential, the developed nanoplatforms could be promising candidates for translational applications, and future in vivo assays could further confirm their potential, so that they might one day be considered as an adjuvant or primary treatment for glioblastoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


