羟烯烃与一氧化碳优化烷氧羰基化合成聚酯及其表征
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
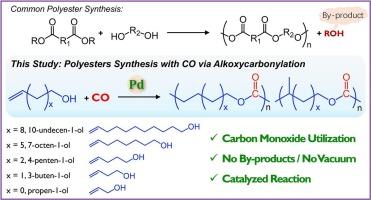
在本研究中,钯催化羟基烯(烯丙醇、3-丁烯-1-醇、4-戊烯-1-醇、7-辛烯-1-醇、10-十一烯-1-醇)的烷氧羰基化反应合成了聚酯。亚甲基链长度决定聚合行为:短链底物(烯丙醇,3-丁烯-1-醇)在分子内环化成内酯,抑制聚合物的形成。相比之下,长链单体(7-辛烯-1-醇,10-十一烯-1-醇)产生高分子量线性聚酯(Mn高达9500)。结构表征通过NMR, SEC, IR和MALDI-TOF-MS证实了主要形成线性聚酯(70-80 mol%),具有较小的分支。机理研究表明,末端烯烃表现出比内烯烃更高的反应活性;然而,钯催化的异构化产生的内部烯烃链末端反应性较低,限制了聚合物的生长。通过优化催化剂组成、聚合温度和单体浓度,提高了聚合物的收率和分子量。这些发现证明了烷氧羰基化将羟基烯烃和CO转化为聚酯的潜力,为单体结构-反应性关系和可持续聚酯合成提供了关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and characterization of polyesters from hydroxyalkenes and CO via optimized alkoxycarbonylation
In this study, polyesters were synthesized via palladium-catalyzed alkoxycarbonylation of hydroxyalkenes (allyl alcohol, 3-buten-1-ol, 4-penten-1-ol, 7-octen-1-ol, 10-undecen-1-ol) using carbon monoxide (CO). The methylene chain length critically dictates the polymerization behavior: short-chain substrates (allyl alcohol, 3-buten-1-ol) undergo dominant intramolecular cyclization to lactones, suppressing polymer formation. In contrast, longer-chain monomers (7-octen-1-ol, 10-undecen-1-ol) yielded high-molecular-weight linear polyesters (Mn up to 9500). Structural characterization using NMR, SEC, IR, and MALDI-TOF-MS confirmed the formation of predominantly linear polyesters (70–80 mol%) with minor branching. Mechanistic studies revealed that terminal olefins exhibit higher reactivity than internal olefins; however, palladium-catalyzed isomerization generates less-reactive internal olefin chain ends, limiting polymer growth. Optimizing the catalyst composition, polymerization temperature, and monomer concentration enhanced the polymer yield and molecular weight. These findings demonstrate the potential of alkoxycarbonylation for converting hydroxyalkenes and CO into polyesters, providing key insights into monomer structure-reactivity relationships and enabling sustainable polyester synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


