聚合物基过渡金属硫化物作为超级电容器电极材料的开发与测试
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
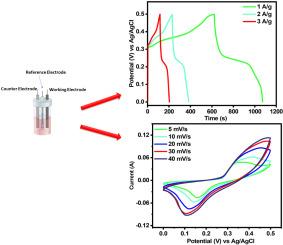
研究人员正在努力开发新的、环保的储能设备,以满足日益增长的能源需求。含聚苯胺的过渡金属硫化物(tms)可以提高超级电容器电极的电化学储能能力。tms有限的能量密度限制了其作为超级电容器电极材料的广泛应用。本工作旨在通过与聚苯胺的纳米复合材料来改善ZrS2的电化学性能。本研究采用水热法制备了ZrS2纳米颗粒凝聚体,制备了具有增强电荷存储能力的ZrS2/聚苯胺。ZrS2/PANI的比电容Cs为1148.31 F/g,而ZrS2在1 a /g时的比电容Cs为701.09 F/g。纳米复合材料优异的电化学性能使其成为氧化还原聚焦电极的优良材料。两电极体系的Cs为197.24 F/g, Pd为900 W/kg,证实了ZrS2/PANI的对称性。在使用3 M KOH的两电极设置中,ZrS2/PANI纳米结构表现出出色的超级电容性能。这是由于聚苯胺的独特特性,它为ZrS2纳米颗粒的生长提供了一个灵活和可扩展的框架。此外,ZrS2/PANI纳米复合材料具有用于超级电容器电极材料的巨大潜力,可以提供更高的Ed (5.54 Wh/kg)和Pd (900 W/kg)。研究结果表明,聚苯胺与ZrS2的结合提高了储能性能,可用于各种储能系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development and testing of polymer-based transition metals sulfide as electrode material for ultracapacitors application
Researchers are working to develop new, eco-friendly energy storing devices to meet the enhancing energy demand. Transition metal sulfide (TMSs) with PANI can enhance supercapacitor electrodes' electrochemical energy storage capacity. The limited energy density of TMSs restricts their widespread use as supercapacitor electrode materials. This work aims to improve the electrochemical performance of ZrS2 through a nanocomposite with PANI. This study fabricated ZrS2/PANI, which exhibits enhanced charge storage capabilities, made up of agglomerated ZrS2 nanoparticles synthesized using hydrothermal methods. ZrS2/PANI showed a specific capacitance (Cs) of 1148.31 F/g, while ZrS2 displayed Cs of 701.09 F/g at 1 A/g. The exceptional electrochemical performance of the nanocomposite makes it an excellent material for redox-focused electrodes. The two-electrode systems exhibit Cs of 197.24 F/g with Pd of 900 W/kg, confirming the symmetric behaviour of ZrS2/PANI. In a 2-electrode setup using 3 M KOH, ZrS2/PANI nanostructures demonstrate outstanding supercapacitive performance. This is due to the unique characteristics of PANI, which provide a flexible and scalable framework for the growth of ZrS2 nanoparticles. Moreover, the ZrS2/PANI nanocomposite has significant potential to be used for supercapacitor electrode material, offering greater Ed (5.54 Wh/kg) with Pd (900 W/kg). The results of this work indicate that the combination of PANI with ZrS2 enhances energy storage performance and can be utilized in various energy storing systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


