城市污泥与有机医疗固体废物共燃烧底渣中重金属形态的研究
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
底渣中的重金属毒性是制约底渣资源化利用的主要问题。本研究收集了城市污泥(MS)和有机医疗固体废物(OMSW)在管式炉中以不同比例共燃的底渣,并分析了Cr、Ni、Cu和Zn的浓度。采用欧共体参考局(BCR)顺序萃取法分析重金属形态特征,在700°C和850°C时,底渣中Cr、Ni和Cu的化学形态以残留态为主,占重金属的57.20 - 77.25%。当反应温度达到1000℃时,Cr的可还原态比例升高最为明显,占35.86 ~ 59.07%,Zn的酸溶态比例最高,占46.65%以上。通过HSC Chemistry 6.0进行的热力学分析表明,温度升高使四种重金属与铁的反应增加,形成相应的高铁酸盐,这解释了1000℃时可还原态比例增加的原因。采用Hakanson潜在生态危害指数法对重金属生态风险进行评价。Zn单项污染因子最高达到高危,Cr、Ni、Cu污染水平均处于高危状态。1000℃时底渣重金属综合潜在生态风险值最高,4种重金属污染程度依次为Cu、Ni、Cr、Zn。本文章由计算机程序翻译,如有差异,请以英文原文为准。
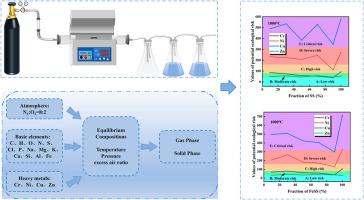
Heavy metal speciation in bottom slags from the co-combustion of municipal sludge and organic medical solid waste
Heavy metal toxicity in bottom slags constitutes a major challenge limiting their reutilization. This study collected bottom slag from co-combustion experiments of municipal sludge (MS) and organic medical solid waste (OMSW) at various ratios in a tubular furnace, followed by analysis of Cr, Ni, Cu, and Zn concentrations. The European Community Bureau of Reference (BCR) sequential extraction method was used to analyze the speciation characteristics of the heavy metals, and the chemical speciation of Cr, Ni, and Cu in the bottom slag at 700 °C and 850 °C was dominated by the residual state, which accounted for 57.20–77.25 % of the heavy metals. When the reaction temperature reached 1000 °C, the proportion of the reducible state of Cr was most obviously elevated, accounting for 35.86–59.07 %, and the proportion of the acid soluble state of Zn was the highest, accounting for more than 46.65 %. Thermodynamic analysis via HSC Chemistry 6.0 demonstrated that increasing the temperature increased the reactions of the four heavy metals with iron to form the corresponding ferrate salts, explaining the increased proportion of the reducible state at 1000 °C. The Hakanson potential ecological hazard index method was used to evaluate the ecological risk of heavy metals. The highest value of the individual pollution factor of Zn reached a high risk, while the pollution levels of Cr, Ni, and Cu were all under elevated risk. The comprehensive potential ecological risk value of the heavy metals in the bottom slag at 1000 °C was the highest, and the pollution levels of the four heavy metals were Cu, Ni, Cr, and Zn in descending order.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


