多组学分析揭示了低硼胁迫下黑氧刺槐茎秆木质素代谢和激素调控的变化
IF 6.8
Q1 PLANT SCIENCES
引用次数: 0
摘要
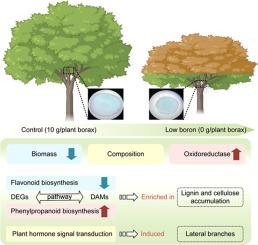
黑胶刺槐(Acacia melanoxylon)是一种珍贵的、用途广泛的常绿树种,具有重要的生态和经济作用;但其对硼胁迫的分子和代谢反应尚未完全阐明。本文采用综合生理、转录组学、代谢组学和激素分析的方法,研究了一年生黑梭梭茎对生长第一年持续施加的慢性低硼胁迫的响应机制。低硼胁迫显著降低了树高和平均节间长,增加了枯枝数。它还诱导了黑梭梭茎中丙二醛(MDA)含量和抗氧化酶活性的显著增加,以及明显的解剖和组成变化。综合转录组学和代谢组学分析显示,低硼胁迫下黑桫椤茎中苯丙素和类黄酮生物合成途径相关基因和代谢物显著富集。此外,激素分析进一步发现生长素水平降低,脱落酸、细胞分裂素、茉莉酸和水杨酸增加。苯丙素和类黄酮生物合成途径的改变以及激素稳态的改变是低硼胁迫下黑梭梭节间缩短的关键反应,促进了主茎侧枝的出现。本研究为木本植物通过次生代谢物与激素相互作用适应低硼胁迫的机制提供了新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multi-omics analysis reveals changes in lignin metabolism and hormonal regulation in Acacia melanoxylon stems under low boron stress
Acacia melanoxylon, a valuable and versatile evergreen tree species, plays a critical ecological and economic role; but its molecular and metabolic responses to boron stress have yet to be completely elucidated. In this paper, we investigated the response mechanisms of one-year-old A. melanoxylon stems to chronic low boron stress imposed continuously throughout their first year of growth, using integrated physiological, transcriptomic, metabolomic, and hormonal analyses. Low boron stress significantly reduced tree height and average internode length while increasing the number of dead branches. It also induced significant increases in malondialdehyde (MDA) content and antioxidant enzyme activity, alongside pronounced anatomical and compositional changes in A. melanoxylon stems. Integrated transcriptomic and metabolomic analyses revealed significant enrichment of genes and metabolites linked to phenylpropanoid and flavonoid biosynthesis pathways in A. melanoxylon stems under low boron stress. Furthermore, hormonal analyses further identified reduced auxin levels alongside increases in abscisic acid, cytokinin, jasmonic acid, and salicylic acid. The alterations in phenylpropanoid and flavonoid biosynthesis pathways, coupled with shifts in hormonal homeostasis are key responses that contribute to internode shortening in A. melanoxylon under low boron stress, facilitating the emergence of lateral branches on the main stem. This study provides novel insights into the mechanisms by which woody plants adapt to low boron stress through the interaction between secondary metabolites and hormones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Plant Stress
PLANT SCIENCES-
CiteScore
5.20
自引率
8.00%
发文量
76
审稿时长
63 days
期刊介绍:
The journal Plant Stress deals with plant (or other photoautotrophs, such as algae, cyanobacteria and lichens) responses to abiotic and biotic stress factors that can result in limited growth and productivity. Such responses can be analyzed and described at a physiological, biochemical and molecular level. Experimental approaches/technologies aiming to improve growth and productivity with a potential for downstream validation under stress conditions will also be considered. Both fundamental and applied research manuscripts are welcome, provided that clear mechanistic hypotheses are made and descriptive approaches are avoided. In addition, high-quality review articles will also be considered, provided they follow a critical approach and stimulate thought for future research avenues.
Plant Stress welcomes high-quality manuscripts related (but not limited) to interactions between plants and:
Lack of water (drought) and excess (flooding),
Salinity stress,
Elevated temperature and/or low temperature (chilling and freezing),
Hypoxia and/or anoxia,
Mineral nutrient excess and/or deficiency,
Heavy metals and/or metalloids,
Plant priming (chemical, biological, physiological, nanomaterial, biostimulant) approaches for improved stress protection,
Viral, phytoplasma, bacterial and fungal plant-pathogen interactions.
The journal welcomes basic and applied research articles, as well as review articles and short communications. All submitted manuscripts will be subject to a thorough peer-reviewing process.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


