甲酸对森林地区和污染城市地区硫酸-氨驱动成核的增强作用
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
甲酸(FA)在大气有机酸中以其普遍存在和结构简单而突出,对大气酸度有重要影响。然而,FA对新颗粒形成(NPF)初级阶段的潜在贡献尚不清楚。本文利用分子动力学(MD)、密度泛函理论(DFT)和大气团簇动力学代码(ACDC)模型对FA参与大气SA(硫酸)-A(氨)团簇的机理进行了研究。MD模拟定性地表明,FA可以与SA和A聚集形成更大的团簇,最大团簇的聚集时间随着温度的降低而缩短。DFT和ACDC结果表明,三元SA-A-FA体系在低温(238.15 K)下热力学更稳定。同时,在低温、高[FA](1011分子/cm3)、低[SA](106分子/cm3)和高[A](1011分子/cm3)区域,FA显著提高了SA-A簇的形成速率。低温NPF机制表明,FA可以通过“催化”机制促进纯SA-A簇的生长,并作为“参与者”在关键簇的形成中发挥不可或缺的作用。这种双重作用不同于我们以前研究中丙二酸和乙醇酸所表现出的“催化”作用。这一发现有助于在FA浓度高的地区,如植被丰富的茂密森林地区,受生物质燃烧影响的地区,或汽车尾气排放和挥发性有机化合物如异戊二烯和萜类化合物释放增加的时期,确定不明原因的NPFs的来源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
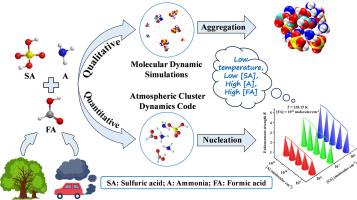
The enhanced role of formic acid on sulfuric acid-ammonia-driven nucleation in forest regions and polluted city areas
Formic acid (FA) is particularly prominent for its ubiquity and structural simplicity among atmospheric organic acids, and exerts a significant influence on atmospheric acidity. However, the potential contribution of FA to the primary stage of new particle formation (NPF) remains unclear. Herein, molecular dynamics (MD), density functional theory (DFT) and the atmospheric cluster dynamics code (ACDC) model have been utilized to evaluate the mechanism of FA participation in atmospheric SA (sulfuric acid)-A (ammonia) clusters. The MD simulations qualitatively suggest that FA can aggregate with SA and A to form larger clusters, and the aggregation time of the largest clusters decreases as the temperature decreases. The DFT and ACDC findings indicate that the ternary SA-A-FA system is thermodynamically more stable at low temperatures (238.15 K). Simultaneously, in regions with low temperatures, high [FA] (1011 molecules/cm3), low [SA] (106 molecules/cm3) and high [A] (1011 molecules/cm3), FA significantly enhances SA-A cluster formation rates. The low-temperature NPF mechanism implies that FA could facilitate the growth of pure SA-A clusters via a “catalytic” mechanism and play an integral role in the genesis of critical clusters as a “participant”. This dual role differs from the “catalytic” role exhibited by malonic and glycolic acids in our previous studies. This discovery could help identify the sources of unexplained NPFs in regions with high FA concentrations, such as densely forested areas with abundant vegetation, regions affected by biomass burning, or periods with elevated vehicle exhaust emissions and the release of volatile organic compounds like isoprene and terpenoids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


