动态菌丝特化重塑使灰葡萄孢在感染过程中克服多种宿主防御
IF 3.3
3区 农林科学
Q2 PLANT SCIENCES
引用次数: 0
摘要
灰霉病是一种坏死性病原体,在田间生产和采后供应链中广泛破坏水果、蔬菜和观赏作物。它可以形成多种特化的菌丝形态,但它们的细胞特征和功能意义尚未得到一致的认识。本研究旨在阐明灰芽孢杆菌的细胞学特征、发育动态和特殊菌丝结构在适应不同抗性水平寄主中的功能作用。利用活细胞成像和电镜技术,证实了灰孢杆菌在分生孢子萌发后形成非典型的附着胞(appressoria-like structures, AP-Ls)。与典型的附着胞不同,ap - l缺乏黑色素沉积,不依赖于分生孢子的自噬。虽然AP-L通常可以介导入侵宿主的初始尝试,但在真菌AP-L结构周围很少观察到植物细胞死亡。随着感染的进展,菌丝尖端可以进一步分化为多细胞感染垫(ICs),其中含有强化的细胞核和细胞器。通过gfp标记的细胞核可视化和组织化学分析,表明与ap - l和未分化的菌丝细胞相比,ic对宿主来源的抗菌化合物表现出更强的耐受性。此外,IC的发育与宿主的抗性水平呈正相关,优先在抗性宿主上形成,并触发IC形成位点附近的局部植物细胞死亡。值得注意的是,atp结合盒转运体BcAtrB对植物抗菌素外排和抗杀菌剂至关重要,在植物感染过程中,它在IC中表达上调,但在camalexin缺陷突变体pen3pdr12中却没有,这一点得到了绿色荧光蛋白报告基因检测的证实,这可能将IC功能与宿主来源的防御化学物质的对抗联系起来。我们的研究结果表明,灰孢杆菌ap - l通常参与初始寄主定植,而多细胞ic则作为适应中心,使真菌能够抵抗来自寄主的强大防御化学物质,因此动态菌丝特化重塑可以很好地支持这种多能病原体成功克服多种植物防御。本文章由计算机程序翻译,如有差异,请以英文原文为准。
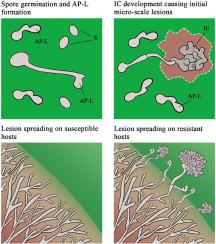
Dynamic hyphal specialization remodeling enables Botrytis cinerea to overcome diverse host defenses during infection
Botrytis cinerea is a necrotrophic pathogen responsible for gray mold disease broadly devastating fruits, vegetables, and ornamental crops both in field production and postharvest supply chains. It can form multiple specialized hyphal morphotypes, but their cellular characteristics and functional significance remain inconsistently recognized. This study aimed to elucidate the cytological characteristics, developmental dynamics, and functional roles of specialized hyphal structures in enabling B. cinerea to adapt to hosts exhibiting varying resistance levels. Utilizing live-cell imaging and electron microscopy, it's demonstrated that B. cinerea forms non-canonical appressoria (termed appressoria-like structures, AP-Ls) after conidial germination. Unlike typical appressoria, AP-Ls lack melanin deposition and are independent of conidial autophagy. Although AP-Ls may normally mediate initial attempts to invade the host, plant cell death could be rarely observed around the fungal AP-L structure. As infection progresses, the hyphal tips could further differentiate into multicellular infection cushions (ICs), which contain intensified nuclei and organelles. Via visualizing the GFP-tagged nuclei and histochemical assays, it's shown that ICs exhibit enhanced tolerance to host-derived antimicrobial compounds than AP-Ls and non-differentiated hyphal cells. Additionally, IC development positively correlates with host resistance levels, being preferentially formed on recalcitrant hosts and triggering localized plant cell death nearby the IC formation sites. Notably, the ATP-binding cassette transporter BcAtrB, critical for phytoalexin efflux and fungicide resistance, appears to be upregulated within ICs during plant infection but not in the camalexin-deficient mutant pen3pdr12 as evidenced by GFP reporter assays, probably linking IC function to counteraction of host-derived defense chemicals. Our findings propose that B. cinerea AP-Ls are normally involved in initial host colonization, while multicellular ICs serve as adaptive hubs enabling the fungus to counteract robust defense chemicals derived from the hosts, thus the dynamic hyphal specialization remodeling could well underpin the success of this generalist pathogen in overcoming diverse plant defenses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.30
自引率
7.40%
发文量
130
审稿时长
38 days
期刊介绍:
Physiological and Molecular Plant Pathology provides an International forum for original research papers, reviews, and commentaries on all aspects of the molecular biology, biochemistry, physiology, histology and cytology, genetics and evolution of plant-microbe interactions.
Papers on all kinds of infective pathogen, including viruses, prokaryotes, fungi, and nematodes, as well as mutualistic organisms such as Rhizobium and mycorrhyzal fungi, are acceptable as long as they have a bearing on the interaction between pathogen and plant.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


