具有工程电荷平衡的自支撑RuO2/NiCo2O4异质结及酸性氧演化的氧化路径机制
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
RuO2作为酸性析氧反应(OER)的活性催化剂,由于其稳定性差和成本高,面临着很大的局限性。在本研究中,我们在碳纸(CFP)上构建了RuO2/NiCo2O4异质结作为酸性OER的高效耐用催化剂。RuO2与NiCo2O4之间的界面相互作用修饰了Ru位点的电子结构,双活性位点的存在激活了氧化路径机制(OPM)。此外,NiCo2O4作为电子储层调节Ru的电荷状态。在反应前,Ru向NiCo2O4的电子转移产生了高价的Ru物质,保证了较高的催化活性。在高电位下,Ni向Ru和Co位点提供电子,保证了材料的稳定性。因此,RuO2/NiCo2O4/CFP只需要187 mV过电位就能达到10 mA cm−2的电流密度,并且在283 h内具有良好的耐久性。本研究提出了一种简单而有效的策略来提高基于RuO2的OER催化剂的质量活性和稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
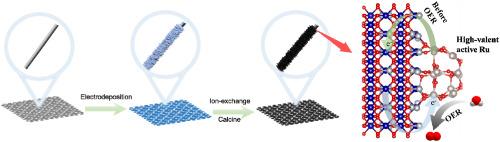
Self-supporting RuO2/NiCo2O4 heterojunction with engineered charge balance and oxide path mechanism for acidic oxygen evolution
RuO2, renowned as the active catalyst for acidic oxygen evolution reaction (OER), faces significant limitations due to its poor stability and high cost. In this study, we constructed RuO2/NiCo2O4 heterojunction on carbon paper (CFP) as an efficient and durable catalyst for acidic OER. The self-supporting structure facilitates enhanced electron and mass transfer, while the interfacial interaction between RuO2 and NiCo2O4 modified the electronic structure of Ru sites, and oxide path mechanism (OPM) was activated due to the exist of dual-active sites. Besides, NiCo2O4 serves as an electron reservoir to regulate the charge state of Ru. Prior to the reaction, electron transfer from Ru to NiCo2O4 generates high-valent Ru species, ensuring high catalytic activity. At high potential, Ni supplies electrons to Ru and Co sites, guaranteeing material stability. As a result, RuO2/NiCo2O4/CFP require only 187 mV overpotential to achieve a current density of 10 mA cm−2, and presenting good durability within 283 h. This work presents a simple yet effective strategy to enhance both the mass activity and stability of RuO2-based OER catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


