结构明确的硫酸软骨素联合SNAC治疗帕金森病的口服疗效
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
帕金森病(PD)是一种进行性神经退行性疾病,治疗选择有限,长期疗效欠佳。虽然糖胺聚糖如硫酸软骨素(CS)具有神经保护潜力,但其临床应用受到口服生物利用度差和靶点特异性有限的限制。在这项研究中,我们首先建立了一种高灵敏度的LC-MS/MS MRM测定方法来定量血浆中的CS。通过动物模型,我们证明与脱氧胆酸(DOCA)修饰相比,与N-[8-(2-羟基苯甲酰)氨基]辛酸酯(SNAC)共给药可显著提高CS的口服生物利用度。随后,我们合成了一种结构明确的6- o-脱硫硫酸软骨素八糖(CS-dp8-de6S),并通过与SNAC共给药评估其对mptp诱导的PD小鼠模型的治疗效果。CS-dp8- de6s /SNAC处理后,大鼠在极悬挂试验中的运动表现得到改善,纹状体多巴胺水平部分恢复,黑质酪氨酸羟化酶表达上调,这些效果显著大于未去硫CS-dp8,表明其特异性增强。凝胶迁移位移法和双重免疫组化的机制研究显示,CS-dp8-de6S治疗可有效减少脑组织纤维蛋白原β链(FGB)的病理沉积。这些发现强调了结构明确的CS低聚糖作为PD的新型疾病改善疗法的潜力,并为开发针对神经退行性疾病中蛋白质病变的碳水化合物策略提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
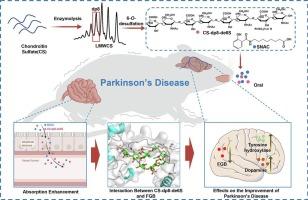
Oral efficacy of structurally defined chondroitin sulfate Co-administered with SNAC in Parkinson's disease
Parkinson's disease (PD) is a progressive neurodegenerative disorder with limited therapeutic options and suboptimal long-term efficacy. Although glycosaminoglycans such as chondroitin sulfate (CS) possess neuroprotective potential, their clinical application is hampered by poor oral bioavailability and limited target specificity. In this study, we first established a highly sensitive LC-MS/MS MRM assay to quantify CS in plasma. Using animal models, we demonstrated that co-administration with N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC) significantly enhanced the oral bioavailability of CS compared to deoxycholic acid (DOCA) modification. Subsequently, we synthesized a structurally defined 6-O-desulfated chondroitin sulfate octasaccharide (CS-dp8-de6S) and evaluated its therapeutic efficacy in MPTP-induced PD mouse models via co-administration with SNAC. Treatment with CS-dp8-de6S/SNAC improved motor performance in the pole and hanging tests, partially restored striatal dopamine levels, and upregulated tyrosine hydroxylase expression in the substantia nigra, these effects were significantly greater than those observed with non-desulfated CS-dp8, demonstrating its enhanced specificity. Mechanistic studies using gel mobility shift assay and dual immunohistochemistry revealed that CS-dp8-de6S treatment effectively reduced pathological fibrinogen β-chain (FGB) deposition in brain tissues. These findings highlight the potential of structurally defined CS oligosaccharides as novel disease-modifying therapeutics for PD and provide a basis for the development of carbohydrate-based strategies targeting proteinopathies in neurodegenerative disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


