邻苯二甲酸二-(2-乙基己基)通过调节IGF-1 m6A甲基化,通过肠道臭杆菌-丁酸轴诱导雌性大鼠子宫内膜异位症
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
子宫内膜异位症是一种复杂的妇科疾病,其特征是子宫内膜组织在子宫腔外的异常生长,对妇女的健康构成了重大挑战。新出现的证据表明,环境污染物,特别是邻苯二甲酸二(2-乙基己基)酯(DEHP),是子宫内膜异位症发展的潜在因素。然而,这种效应背后的精确分子机制仍然知之甚少。在此,我们在大鼠模型中研究了肠臭杆菌/丁酸介导的m6A甲基化在dehp诱导的上皮-间质转化(EMT)和子宫内膜异位症中METTL3/IGF-1信号通路中的作用。我们的研究表明,DEHP暴露会改变肠道微生物群组成,导致mettl3介导的胰岛素样生长因子1 (IGF-1)通路中m6A修饰的调节。这种修饰增强了子宫内膜细胞的EMT,促进了子宫内膜异位症病变的形成。我们采用多层方法,包括16S rRNA测序、靶向代谢组学、MeRIP-seq、定量聚合酶链反应、western blotting和免疫组织化学,来阐明肠道臭杆菌/丁酸途径介导的METTL3/IGF-1 m6A修饰在dehp诱导的子宫内膜异位症中的机制作用。结果显示,dehp暴露大鼠的微生物多样性发生了显著变化,METTL3/IGF-1 m6A甲基化相应增加,这与E-cadherin和N-cadherin等EMT标记物直接相关。我们的研究结果揭示了一种新的肠道微生物介导的机制,DEHP暴露通过m6A甲基化驱动子宫内膜异位症,为该疾病的环境和分子基础提供了有价值的见解。这项研究不仅促进了我们对DEHP在子宫内膜异位症发病机制中的作用的理解,而且还提示了一个假定的肠道臭杆菌-丁酸盐- mettl3 /IGF-1轴可能有助于疾病进展。然而,这些关联仍然是相关的,因果关系需要通过功能实验进一步验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
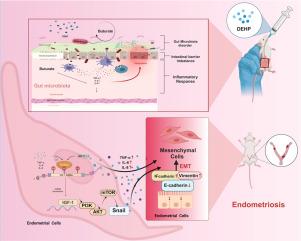
Di-(2-ethylhexyl) phthalate induces endometriosis by modulating IGF-1 m6A methylation via the intestinal Odoribacter–butyric acid axis in female rats
Endometriosis, a complex gynecological disorder characterized by aberrant growth of endometrial tissue outside the uterine cavity, poses a significant challenge to women's health. Emerging evidence implicates environmental pollutants, particularly di-(2-ethylhexyl) phthalate (DEHP), as potential contributors to endometriosis development. However, the precise molecular mechanisms underlying this effect remain poorly understood. Herein, we investigated the role of intestinal Odoribacter/butyric acid-mediated m6A methylation in METTL3/IGF-1 signaling in DEHP-induced epithelial-mesenchymal transition (EMT) and endometriosis in a rat model. Our study demonstrated that DEHP exposure alters the gut microbiota composition, leading to modulation of METTL3-mediated m6A modification in the insulin-like growth factor 1 (IGF-1) pathway. This modification enhances EMT in endometrial cells and promotes endometriotic lesion formation. We used a multi-layered approach, including 16S rRNA sequencing, targeted metabolomics, MeRIP-seq, quantitative polymerase chain reaction, western blotting, and immunohistochemistry, to elucidate the mechanistic role of intestinal Odoribacter/butyric acid pathway-mediated METTL3/IGF-1 m6A modification in DEHP-induced endometriosis. The results revealed a significant shift in microbial diversity and a corresponding increase in METTL3/IGF-1 m6A methylation in DEHP-exposed rats, which was directly linked to EMT markers such as E-cadherin and N-cadherin. Our findings reveal a novel gut microbiota-mediated mechanism by which DEHP exposure drives endometriosis via m6A methylation, providing valuable insights into the environmental and molecular basis of the disease. This study not only advances our understanding of the role of DEHP in endometriosis pathogenesis, but also suggests a putative intestinal Odoribacter–butyrate–METTL3/IGF-1 axis that may contribute to disease progression. However, these associations remain correlative, and causality requires further validation through functional experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


