病理综合多模式模型评价不可切除肝内胆管癌化疗免疫治疗疗效
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景:aimschemo免疫疗法已成为不可切除肝内胆管癌(ICC)的一线治疗方法。然而,在不到30%的患者中观察到持久的临床反应,需要生存获益的生物标志物。因此,本研究的目的是为接受化疗免疫治疗的ICC患者开发和验证基于病理的预后特征。方法本多中心研究纳入有活检标本的ICC患者。从数字H&; e染色图像中提取病理特征,并通过机器学习(ML)开发ICC的病理特征(PS-ICC)。SHapley Additive explained提供算法解释,The Cancer Genome Atlas数据库支持生物学解释。我们探讨了PS-ICC作为替代指标与放射反应比较的潜力。结果在预处理标本的基础上,共纳入189例化疗免疫治疗患者。单因素Cox分析显示,病理驱动的ML模型的PS-ICC状态与接受化疗免疫治疗的不可切除ICC患者的总生存率(OS)相关(培训队列:风险比(HR) = 0.09, 95% CI, 0.05-0.14, p <0.001;验证队列:HR = 0.20, 95% CI 0.08-0.47, p <0.001)。PS-ICC具有肿瘤微环境异质性特征,较低的评分与M0巨噬细胞浸润减少(p = 0.043)、程序性死亡配体1表达和程序性死亡-1检查点途径减少(p = 0.002)相关。此外,与治疗后3个月的实体瘤版本1.1应答评价标准相比,PS-ICC状态可以作为OS的替代标志物(培训队列:1年AUC: 0.868 vs 0.701; 2年AUC: 0.787 vs 0.694)。ps - icc综合nomogram预后预测模型在准确性上优于传统预测模型(一致性指数:0.80 vs. 0.71)。结论PS-ICC具有作为化疗免疫治疗ICC患者生存预测的替代终点的潜力,其肿瘤微环境相关性证明了其生物学可行性。有必要进行前瞻性试验以确认临床应用。影响和意义本研究开发并验证了一种基于机器学习的病理特征,可以准确预测接受化学免疫治疗的肝内胆管癌(ICC)患者的总生存率。ICC的病理特征提供了一种基于生物学的预处理生物标志物,用于对化疗免疫治疗的患者进行分层,潜在地减少过度治疗并指导个性化策略。通过证明与总生存期的强相关性,这一特征可以作为临床试验的替代终点,从而加速药物开发。此外,它与免疫途径的联系可以为ICC提供新的治疗靶点。然而,临床应用需要前瞻性验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
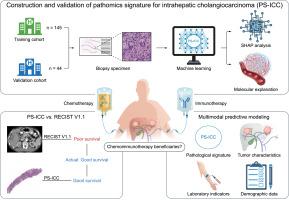
A pathomics-integrated multimodal model to evaluate chemoimmunotherapy efficacy in unresectable intrahepatic cholangiocarcinoma
Background & Aims
Chemoimmunotherapy has emerged as the first-line therapy for unresectable intrahepatic cholangiocarcinoma (ICC). However, durable clinical responses are observed in less than 30% of patients, necessitating biomarkers of survival benefit. Thus, the aim of this study was to develop and validate a pathomics-based prognostic signature for patients with ICC receiving chemoimmunotherapy.
Methods
This multicenter study included patients with ICC with biopsy samples. Pathomics features were extracted from digital H&E-stained images, and the pathomics signature for ICC (PS-ICC) was developed by machine learning (ML). SHapley Additive exPlanations provided algorithmic explanation, and The Cancer Genome Atlas database supported biological interpretation. We explored the potential of PS-ICC as surrogate index compared with radiological response.
Results
Based on pretreatment specimens, 189 patients receiving chemoimmunotherapy were included. Univariate Cox analysis demonstrated that the PS-ICC status from a pathomics-driven ML model was associated with overall survival (OS) in patients with unresectable ICC undergoing chemoimmunotherapy (training cohort: hazard ratio (HR) = 0.09, 95% CI, 0.05–0.14, p <0.001; validation cohort: HR = 0.20, 95% CI 0.08–0.47, p <0.001). PS-ICC characterized tumor microenvironment heterogeneity, with lower scores correlating with reduced M0 macrophage infiltration (p = 0.043) and programmed death-ligand 1 expression and programmed death-1 checkpoint pathways (p = 0.002). In addition, PS-ICC status could serve as a surrogate marker for OS compared with the Response Evaluation Criteria in Solid Tumors Version1.1 at 3 months post treatment (training cohort: AUC at 1 year: 0.868 vs. 0.701; 2 year: 0.787 vs. 0.694). The PS-ICC-integrated nomogram outperformed conventional prognostic model in accuracy (Concordance index: 0.80 vs. 0.71).
Conclusions
The PS-ICC demonstrates potential as a surrogate endpoint for survival prediction in patients with ICC undergoing chemoimmunotherapy, with biological plausibility evidenced by its tumor microenvironment associations. Prospective trials are warranted to confirm clinical utility.
Impact and implications
This study developed and validated a machine learning-based pathomics signature that accurately predicts overall survival in patients with intrahepatic cholangiocarcinoma (ICC) receiving chemoimmunotherapy. The pathomics signature for ICC provides a biologically grounded, pretreatment biomarker to stratify patients for chemoimmunotherapy, potentially reducing overtreatment and guiding personalized strategies. By demonstrating strong correlation with overall survival, this signature could serve as a surrogate endpoint in clinical trials, thereby accelerating drug development. Furthermore, its link to immune pathways could inform novel therapeutic targets in ICC. However, prospective validation is needed for clinical adoption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


