壳聚糖基羧甲基壳聚糖聚电解质复合膜与壳聚糖作为锌离子电池中抑制锌枝晶的替代隔膜
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锌离子电池(zib)由于能量密度高、环保、易于加工等优点,作为替代能源储存装置的使用正在增加。然而,性能受到锌枝晶形成等挑战的限制。本文研究了由羧甲基壳聚糖(CMCS)和壳聚糖(CS)制成的聚电解质复合膜(chi-PEC)作为抑制枝晶生长的隔膜。CMCS和CS混合形成白色沉淀,然后离心压缩成膜。在膜形成过程中加入盐掺杂,评价其效果。膜依赖于动态离子交联-没有化学交联剂-在CMCS和CS官能团之间,这促进了均匀的锌离子分布和最大限度地减少离子排斥。CMCS/CS膜的最佳离子电导率约为3.71 × 10−3 S cm−1,锌的转移数约为0.52。经过200次充放电循环后,chi-PEC隔膜的锌阳极呈现致密的锌沉积,表明枝晶形成减少,电池稳定性增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
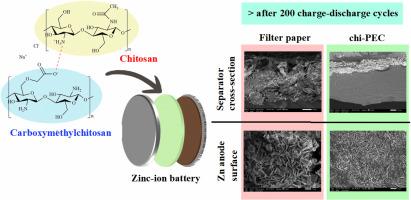
Chitosan-based polyelectrolyte complex membrane of carboxymethylchitosan and chitosan as alternative separator for zinc dendrite suppression in zinc-ion batteries
The use of zinc-ion batteries (ZIBs) as alternative energy storage devices is increasing owing to the high energy density, eco-friendliness, and ease of processing. However, the performance is limited by challenges such as zinc dendrite formation. Herein, polyelectrolyte complex membranes made from carboxymethylchitosan (CMCS) and chitosan (CS), denoted as chi-PEC, were developed as separators to suppress dendrite growth. CMCS and CS were mixed to form white precipitates, which were then centrifuged and compressed into membranes. Salt doping was applied during membrane formation to evaluate its effects. The membranes rely on dynamic ionic crosslinking—without chemical crosslinkers—between CMCS and CS functional groups, which promotes uniform zinc-ion distribution and minimizes ionic repulsion. The CMCS/CS membranes exhibited optimum conductivity of ion on approximately of 3.71 × 10−3 S cm−1 and a transference number of zinc of around 0.52. After 200 charge–discharge cycles, zinc anodes with chi-PEC separators showed dense zinc deposition, indicating reduced dendrite formation and enhanced battery stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


