高温燃料电池和碱性电解水用含支链萘环聚苯并咪唑膜
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
聚合物的离子电导率对能量转换装置的工作效率有着深远的影响。电导率的提高有效地降低了欧姆电阻,从而优化了这些系统的整体功能。先前的研究表明,将萘环纳入聚苯并咪唑(PBI)分子结构中,可以使材料具有优异的氧化稳定性。在这项工作中,低含量的大3D结构和高刚性的金刚烷结构作为分支点引入到含萘的PBI中,使其能够吸收更多的电解质,同时提高尺寸稳定性。具体来说,在磷酸(PA)吸收下,支链1.0Ad - NPBI膜的质子电导率高达155.8 mS cm - 1。在H2 - O2燃料电池(0.6 mg cm - 2 Pt)中,在180°C时达到1022.4 mW cm - 2的峰值功率密度。吸收KOH溶液后,其氢氧根离子电导率达到176.7 mS cm - 1,在碱性电解水(AWE)电池中(在6 M KOH中,在80°C下),在2.0 V下电流密度为5.4 a cm - 2。这证实了分支是一种非常适合改善PBI性能的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
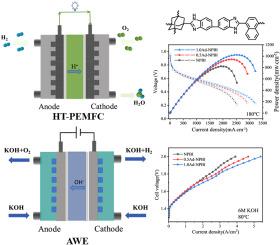
Branched naphthalene ring-containing polybenzimidazole membranes for high-temperature fuel cells and alkaline electrolytic waters
The ionic conductivity of polymers exerts a profound influence on the operational efficiency of energy conversion devices. Elevated conductivity efficiently mitigates ohmic resistance, thus optimizing the overall functionality of these systems. Previous research has demonstrated that incorporating naphthalene rings into polybenzimidazole (PBI) molecular structures confers exceptional oxidative stability to the material. In this work, low−content large 3D structures and highly rigid adamantane structures are introduced as branching points into the naphthalene−containing PBI, enabling it to absorb more electrolytes while improving dimensional stability. Specifically, upon phosphoric acid (PA) absorption, the branched 1.0Ad−NPBI membrane exhibited a proton conductivity of up to 155.8 mS cm−1. In an H2−O2 fuel cell (0.6 mg cm−2 Pt), it achieved a peak power density of 1022.4 mW cm−2 at 180 °C. After absorbing KOH solution, its hydroxide ion conductivity reached 176.7 mS cm−1, and it delivered a current density of 5.4 A cm−2 at 2.0 V in alkaline electrolytic water (AWE) cells (in 6 M KOH, at 80 °C). It confirms that branching is a very suitable strategy for improving the performance of PBI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


