石墨和纳米碳的钠离子存储性能:钠二甘胺共嵌入石墨烯纳米片
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
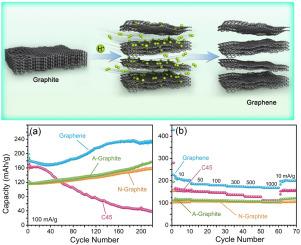
本研究探讨了各种碳材料的钠离子(na离子)存储性能,包括市售的碳纳米颗粒(C45),天然和人造石墨,以及通过绿色可持续熔盐剥落法生产的少层石墨烯。这些材料的结构、微观结构、电学和电化学特性在酯基(碳酸盐)和醚基(二莱姆)电解质中进行了评估。在传统的酯电解质中,钠离子插入石墨和石墨烯仍然受到限制,而在醚基体系中,与二甘醚共插入是非常有效的。在所研究的材料中,少层石墨烯表现出最有前景的电化学性能,在100 mA/g下循环220次后,其容量增加到235 mAh/g,在150次循环后,na离子扩散达到8.4 × 10−11 cm2/s。这一性能归功于石墨烯卓越的导电性(在6.2 MPa下为1336 S/m)及其纳米层状结构,这有助于形成稳定的na -二糖嵌入化合物。原位充电/放电-拉曼光谱证实了这一机制,揭示了0.58-0.63 V(放电)和0.66-0.80 V(充电)的电压平台,以及1456和1461 cm−1的拉曼特征。这些发现证明了乙醚相容石墨烯基阳极具有很高的电化学潜力,可用于可持续的钠离子存储。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Na-ion storage performance of graphite and nanocarbons: Na-diglyme co-intercalation into graphene nanosheets
This study explores the sodium-ion (Na-ion) storage performance of various carbon materials, including commercially available carbon nanoparticles (C45), natural and artificial graphite, and few-layer graphene produced through a green and sustainable molten salt exfoliation method. Structural, microstructural, electrical and electrochemical characteristics of these materials are evaluated in both ester-based (carbonate) and ether-based (diglyme) electrolytes. While Na-ion intercalation into graphite and graphene remains limited in conventional ester electrolytes, co-intercalation with diglyme in ether-based systems is highly effective. Among the materials studied, the few-layer graphene exhibits the most promising electrochemical performance, delivering a rising capacity of 235 mAh/g after 220 cycles at 100 mA/g, with Na-ion diffusion reaching 8.4 × 10−11 cm2/s after 150 cycles. This performance is attributed to graphene's exceptional electrical conductivity (1336 S/m at 6.2 MPa) and its nano-layered structure, which facilitates the formation of stable Na-diglyme intercalation compounds. In situ charge/discharge-Raman spectroscopy confirms this mechanism, revealing voltage plateaus at 0.58–0.63 V (discharge) and 0.66–0.80 V (charge), along with Raman features at 1456 and 1461 cm−1. These findings demonstrate the high electrochemical potential of ether-compatible graphene-based anode for sustainable Na-ion storage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


