钠离子电池中完全固溶反应和可逆阴离子氧化还原化学的局部结构调节和电荷补偿
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于其高能量密度和天然丰度,层状铁/锰基氧化物被认为是有前途的可持续储能阴极候选者,但在高压循环过程中稳定阳离子(过渡金属)和阴离子(晶格氧)氧化还原反应面临着严峻的挑战。为了解决这些问题,我们设计了Cu/B共掺杂的p2型Na0.7Fe0.2Cu0.1Li0.1B0.02Mn0.6O2 (NFCLBM)阴极。Cu-O和B-O共价键的协同作用增强了氧框架的刚性,有效地抑制了高压下的O2 -过氧化。同时,Cu掺杂激活了Mn/O电荷补偿机制,减轻了氧损失引起的容量衰减,同时提高了空气稳定性。因此,NFCLBM在1c下提供135.5 mAh g - 1的初始容量,并在200次循环后保持84.58%的容量保留率。原位x射线衍射揭示了NFCLBM充放电过程中完整的固溶反应机理,表明其具有良好的结构稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
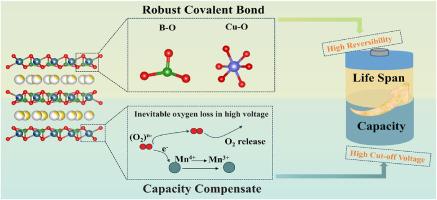
Complete solid-solution reaction and reversible anionic redox chemistry in sodium-ion batteries enabled by local structure regulation and charge compensation
Layered iron/manganese-based oxides, recognized as promising cathode candidates for sustainable energy storage due to their high energy density and natural abundance, face critical challenges in stabilizing cationic (transition metal) and anionic (lattice oxygen) redox reactions during high-voltage cycling. To address these issues, we designed a Cu/B co-doped P2-type Na0.7Fe0.2Cu0.1Li0.1B0.02Mn0.6O2 (NFCLBM) cathode. The synergistic effects of robust Cu-O and B-O covalent bonds enhance oxygen framework rigidity, effectively suppressing O2− overoxidation at high voltages. Concurrently, Cu doping activates a Mn/O charge compensation mechanism, mitigating capacity decay caused by oxygen loss while improving air stability. Consequently, NFCLBM delivers an initial capacity of 135.5 mAh g−1 at 1 C and maintains 84.58 % capacity retention after 200 cycles. In situ X-ray diffraction reveals the complete solid solution reaction mechanism of NFCLBM during charging and discharging, indicating its excellent structural stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


