原位氮化用于析氢反应的碳基Ni3N电催化剂:合成和机理见解
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
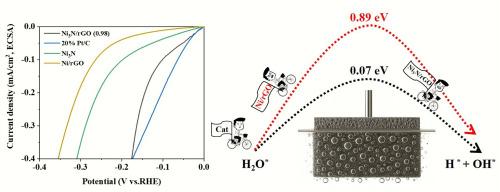
氮化镍(Ni3N)在碱性介质中表现出良好的析氢电化学活性。然而,在不使用氨气氛或混合胺基前体的情况下合成Ni3N仍未得到探索,碳基Ni3N增强HER催化性能的机制仍未完全了解。本文首次报道了一种简单、环保的原位氮化直接加热合成Ni3N/rGO电催化剂的方法,并展示了其作为高效HER电极材料的应用。表征结果表明,C4K2N4Ni·xH2O同时作为氮源和镍源。水热反应后,催化活性位点通过离子交换均匀分散在rGO表面,在氮气气氛下加热成功合成了均相Ni3N/rGO。rGO基质增强了催化活性位点与电解质之间的接触,即使在极低的金属负载下(Ni3N/rGO(0.98)在−10 mA/cm2时为195 mV,在−80 mA/cm2时为305 mV)也能实现优异的HER性能。第一性原理密度泛函理论(DFT)计算表明,Ni3N/rGO电催化剂具有较低的自由能和较低的反应能垒。在优化条件下,Ni3N/rGO(0.98)的过电位(ecsa标准化)与20% Pt/C的过电位相当,并且在高电流密度的循环测试中具有出色的稳定性。此外,取代阴离子交换树脂作为碳源也可以获得类似的催化性能。所提出的碳基Ni3N电催化剂的合成策略为高性能HER电极材料的制造提供了一种可扩展的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In situ nitridation of carbon-based Ni3N electrocatalysts for the hydrogen evolution reaction: Synthesis and mechanistic insights
Nickel nitride (Ni3N) exhibits promising electrochemical activity for the hydrogen evolution reaction (HER) in alkaline media. However, the synthesis of Ni3N without employing an ammonia atmosphere or mixed amine-based precursors remains unexplored, and the mechanism by which carbon-based Ni3N enhances HER catalytic performance is still not fully understood. Herein, we report for the first time a simple and environmentally friendly in situ nitridation method to synthesize a Ni3N/rGO electrocatalyst via direct heating, and demonstrate its application as an efficient HER electrode material. Characterization results show that C4K2N4Ni·xH2O serves as both the nitrogen and nickel source. Following a hydrothermal reaction, the catalytically active sites are uniformly dispersed on the rGO surface via ion exchange, and homogeneous Ni3N/rGO is successfully synthesized through heating under a nitrogen atmosphere. The rGO matrix enhances contact between the catalytic active sites and the electrolyte, enabling excellent HER performance even with extremely low metal loading (Ni3N/rGO (0.98) are 195 mV at −10 mA/cm2, and 305 mV at −80 mA/cm2). First-principles density functional theory (DFT) calculations reveal that the Ni3N/rGO electrocatalyst possesses lower free energy and a reduced reaction energy barrier. Under optimized conditions, Ni3N/rGO (0.98) exhibits a comparable overpotential (ECSA-normalized) to that of commercial 20 % Pt/C, along with outstanding stability during cyclic testing at high current densities. Moreover, substituting anion exchange resin as the carbon source also yields similar catalytic performance. The proposed synthesis strategy for carbon-based Ni3N electrocatalysts offers a scalable approach for the fabrication of high-performance HER electrode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


